Medical Accessory & Diagnostic Industry MagazineMDDI Commodity Index

An MD&DI October 1999 Column
Ever aback the reengineering efforts at FDA’s Center for Accessories and Radiological Health (CDRH) began in 1997, at atomic one inspection-related aggregation has been in place. The aboriginal reengineering activities, however, targeted alone the issues of pre- and postinspection efficiencies. CDRH was, in fact, nibbling about the edges of the absolute issue: how to change the assay activity itself. Certainly, the anticipation of reengineering a aloft assay diplomacy represented a alarming task.
The charge for change was substantial. Abundant and cogent issues bare to be addressed. The activity for medical accessory inspections had not undergone a aloft change in years. One acumen to attending at reengineering the assay activity was the deepening of FDA’s advantage of the accessory industry. Instead of analytical accessory firms every two years, the bureau begin itself abutting the achievability of inspections every seven years. Indeed, admitting the “inventory” of registered accessory firms was increasing, the accessible cardinal of investigator hours to achieve the inspections was decreasing. In adjustment to assay the beyond account with beneath investigators, the bureau bare a beneath inspection. At the aforementioned time, the activity alleged for affection inspections, with the bureau absorption on the key areas of the industry’s affection systems.
Another cogent bang for reengineering was the charge for FDA to accede the harmonization of inspections with added government entities. The alternate acceptance acceding (MRA) alive by Admiral Clinton in December 1998 stipulates that the U.S. government will commonly endorse assay belletrist from conformity-assessment bodies in the European Union by December 2001.
Finally, the bureau accomplished that the absolute activity for analytical firms was not in alignment with the affection arrangement (QS) regulation. FDA was application an assay abode congenital about the apriorism that firms apparently had accessory problems and that it was the job of FDA to acquisition those problems. Indeed, over time there accept been problems involving acceptable accomplishment practices (GMPs). For several years, affection arrangement assay abstracts accept apparent an access in “official activity indicated” notations. Such allegation usually aftereffect in admonishing belletrist or added accomplishments taken by the agency.
While the albatross for demonstrating acquiescence has consistently been on the manufacturers, things afflicted with the actualization of the QS regulation. The regulation, which becamse able in June 1997, is absolute specfic in captivation administration amenable for affection arrangement performance.The artifice of ISO-type requirements that came into abode with the QSR meant that administration of accessory firms now accept to ensure that able and able affection systems are accustomed and maintained. As firms accessory up to accommodated the regulation, FDA has assured that it needs an assay activity that mirrors this new reality.
ACCOMPLISHING CHANGE
The reengineering activity complex a cardinal of key stages including the accumulation of teams, the abode and assay of architecture inputs from assorted sources, the alertness of a alive model, and the final addition of a new access to affection arrangement inspections.
Team Formation. FDA’s reengineering aggregation was formed adjoin the end of 1997. Application national, district, and CDRH accessory experts, the aggregation brought cogent adeptness to the table. Adopting the reengineering procedures that had been activated and accurate in Michael Hammer’s book Reengineering the Corporation enabled FDA to convention a adjustable and accessible process.
Whereas commonly the bureau would sit bottomward and carbon its own assay process, with reengineering, all-encompassing stakeholder input, benchmarking, and architecture controls were alive in developing a new process. Proposals were put afore the accessible and the industry aboriginal and throughout the project. An ad hoc accumulation was formed absolute accessory manufacturers, FDA, and added industry experts. The accumulation met several times at breezy diplomacy hosted by FDLI in Washington, DC, to altercate how a affection arrangement assay could and should be done.
Design Inputs. The aboriginal ascribe into the activity was the advancement by industry that, during affection arrangement inspections, FDA bare to accent the accent of administration in establishing an able affection system. Addition ascribe came from the adventures of firms that are audited by notified bodies and capital FDA to use some of the aforementioned procedures. Finally, FDA and industry accomplished that, beneath the absolute assay process, it was not aberrant for firms to abode affection issues brought to their absorption through FDA inspections. However, the companies would not necessarily booty albatross for those problems, or attending at them from a systems viewpoint.
The Model. Alive with the ad hoc group, FDA developed a new assay activity that appeared to accommodated all user needs. After several diplomacy with the group, FDA apparent the activity at an accessible accessible affair in Rockville, MD, in May 1998. The new assay process—called the affection arrangement assay technique, or QSIT—was arise to the accessible via the reengineering Web armpit in September 1998 in a certificate alleged the QSIT Handbook, the appellation of which was after afflicted to Adviser to Inspections of Affection Systems.
A New Assay Approach. The QSIT activity encompasses three new approaches that are altered from the acceptable affection arrangement inspection. The aboriginal is the abstraction of a “top-down” process. With acceptable inspections—and for those that will abide in the approaching beneath the “for-cause” approach—the investigator begins the activity by attractive at raw abstracts or annal that chronicle to accessory problems and assembly issues. These “bottom-up” inspections assignment their way up the system, with the ambition of extensive the basis account of the problem(s) and alive out a band-aid with the firm. With QSIT, the assay begins with assay of top systemwide procedures and policies. As added admonition is needed, the investigator bores bottomward further into the records, demography a top-down approach.
The added new access is the abstraction of almanac sampling. Sampling allows the investigator to attending at a alternative of annal by application statistical tables as a adviser for free how abounding abstracts to examine. This helps to accumulate the activity moving, and to awning added arena during the inspection.
The final new access is the abstraction of preinspection almanac review. With QSIT, board are encouraged to ask for annal afore the assay absolutely begins. While firms are not adapted to accelerate any annal to FDA above-mentioned to the absolute alpha of the inspection, abstracts from the QSIT abstraction announce that this convenance adored time and added the adeptness of the inspection.

QSIT PRINCIPLES
Two aspects of QSIT analyze it as a different assay process: the attempt of administration albatross and of antidotal and antitoxin accomplishments (CAPA). The assumption of administration albatross bureau that QSIT places a aloft accent on evaluating a firm’s administration and how able-bodied it is accomplishing in establishing an able affection system. QSIT inspections both alpha and accomplishment with a assay of the firm’s administration controls. The QSIT access absolutely advises the investigator to accede the actuality that product, design, and CAPA problems begin during the assay can be acclimated to tie aback to the disability of the firm’s administration to authorize an able affection system.
The assumption of CAPA involves a new way for the investigator to attending at the firm’s systems for administration CAPA information. The investigator is acquainted that, beneath QSIT, it is accessible for artefact and activity nonconformances to occur, aloof as they would beneath any system. But what is added important is the firm’s activity for administration nonconformance data: the board are told that the arrangement for administration affection abstracts is added important than the absolute contest that abide in the data. Because the board are blockage the system, QSIT will aftereffect in added abstracts actuality fabricated about the arrangement that will heavily access FDA assay decisions. It appropriately becomes abnormally benign for companies to authorize and beforehand acceptable systems above-mentioned to inspection. A account of the QSIT access is that it should aftereffect in the dialogues amid the bureau and the firms actuality on systems issues, rather than on issues of artefact and activity problems.
Quality Subsystems. QSIT is an assay activity based on the subsystems of the affection system. It has been adapted that a affection arrangement can be burst bottomward into seven subsystems (Figure 1). To beforehand the adeptness of the assay process, the QSIT access focuses on the four primary subsystems: administration controls, architecture controls, antidotal and antitoxin actions, and assembly and activity controls. The added three subsystems—material controls; records, documents, and change controls; and accessories and accessories controls—are addressed by QSIT through linkages rather than through specific coverage. As adumbrated in the guide, linkages acquiesce the investigator to leave a subsystem in adjustment to awning addition aspect of the affection system. They are acclimated because subsystem probes can not be performed absolute of added aspects of the system.
Figure 1. This schematic of affection arrangement subsystems emphasizes the axial accent placed by QSIT on administration controls.
As mentioned above, QSIT places administration controls at the affection of the affection system. However, the abstraction of CAPA—as the breadth for all affection data—is additionally absolute important.
Subsystem Inspection. The Adviser to Inspections of Affection Systems directs the QSIT assay activity and sets alternating a compatible alignment for analytical anniversary of the subsystems. For anniversary subsystem, the guidebook contains four accepted sections: purpose and importance, assay objectives, flowcharts for allegorical the assay process, and a narrative, accouterment how-to admonition on analytical anniversary subsystem. Sampling diplomacy and instructions are additionally provided in the guide, which can be begin in its absoluteness (in PDF) on the QSIT Web site.
The QSIT “Establish” Test. The QSIT access uses what has been alleged the “establish” assay in anniversary of the subsystem probes. What this bureau is that anniversary of the four subsystem inspections gain according to the three compenents of the regulation’s analogue of establish: define, document, and apparatus (21 CFR 820.3(k)).
The inspections alpha with an assay of the abstracts that the abutting uses to ascertain and certificate the subsystem. These abstracts are the firm’s accepted operating procedures (SOPs) and behavior that chronicle to the subsystem. For example, during the assay of the architecture ascendancy subsystem, the absolute aboriginal QSIT cold is to attending at the company’s procedures for architecture controls.
The added allotment of the QSIT authorize assay is the appraisal of what the abutting is accomplishing to accumulate the subsystem in place. This appraisal is absolutely two abstracted reviews. The aboriginal determines whether the abutting is accomplishing what its procedures say it is declared to be doing. The added evaluates whether the processes actuality performed accede with the regulation. Samples of annal will be acclimated to verify acquiescence in several aspects of the review.
The third allotment of the QSIT authorize assay is the appraisal of whether the abutting is accustomed out the activity for that subsystem adequately. Various annal will be brash to actuate adequacy. For example, beneath CAPA, the investigator will actuate if the CAP subsystem is capturing and acting on affection data. If it fails to do so, the subsystem could be accounted inadequate.
As in any inspection, QSIT challenges a arrangement by acid annal and comparing the allegation adjoin procedures and regulations. This aspect of the assay is the breadth in which QSIT is abutting to the old assay procedures. In this regard, FDA has approved ample adeptness in evaluating the processes of manufacturers. For example, during a bottom-up inspection, the investigator will analysis raw abstracts (records) and analyze the admonition in those annal adjoin the firm’s procedures and the regulations. Although the bureau has had abundant success in analytical via this process, the capacity were acutely time-consuming and board had the difficult assignment of arch aback they had abundant abstracts to announce a abutting was or was not in compliance. The use of sampling tables with QSIT makes this assignment abundant easier.
Sampling Tables. One of the strengths of QSIT is the sampling tables. These tables acquiesce an investigator to awning added arena during a QSIT assay than what adeptness accept been covered traditionally. The sampling tables acquiesce the investigator to certificate the actuality of problems (or their nonexistence) through statistically accurate sampling.
In a acceptable inspection, anniversary investigator had to attending at annal and adjudge how abounding examples of acquiescence or contravention were bare to amuse his or her administrator and acquiescence officer. Was one almanac enough? Fifty? Five hundred? Generally, the investigator had to accomplish abiding that abundant annal had been examined, accustomed the time and accomplishment complex in abiding to the abutting for added documents. Obtaining the adapted annal was addition problem. Did the investigator certificate the affliction or best case? How and why did he or she accept those records? With the QSIT access and the use of sampling tables, the investigator is led to the adapted annal and is provided admonition on how abounding annal to attending at to actuate and certificate a abuse or nonviolation.

Table I. Archetype of sampling diplomacy and high aplomb levels (95% aplomb limit).
The tables are acclimated to acquaint the bureau the likelihood that a abuse will occur. For example, Table I shows that if you attending at 35 annal and acquisition no examples of the abuse you are attractive for, you can be 95% assured that the citizenry of annal from which you drew the sample has that abuse in no greater than 10% of the records. An important point about the sampling statistics is that the tables can be acclimated to announce both the anticipation of the abuse occurring in the citizenry and the anticipation of the abuse not occurring.
Order of Systems Inspections. QSIT guides the investigator through the four aloft subsystems in a assigned manner. The assay starts with administration controls and gain to architecture controls, CAPA, and assembly and activity controls, finishing with a acknowledgment to administration controls. While these probes into the affection arrangement may arise to be alert evaluations of specific areas, in absoluteness it is neither applied nor acceptable that the inspections will chase the activity so closely. In fact, the activating activity of establishing affection systems additionally after-effects in a activating activity for inspections.
As the investigator goes bottomward one of the four probes, linkages that tie aback to the added subsystems will beforehand the assay out of the appointed subsystem and into added sub-systems as needed. For example, during the delving of assembly and activity controls, the investigator is asked to sample training annal of assembly employees, and training is technically covered beneath the administration breadth of the regulation. It is appropriately not accessible to conduct the four probes in absolute abreast from one another.
During the accepted baseline QSIT inspection, the bulk of time spent in anniversary subsystem varies from a bisected day to about two days. There is no accustomed time absolute for anniversary subsystem review; rather, the cardinal of annal brash will actuate the breadth of time anniversary requires. An boilerplate of one day per subsystem is adapted in the guide, but some (CAPA, for example) are accepted to booty best and others (such as management) shorter. The probes will booty best if the investigator selects beyond sample sizes from the sampling tables. Aback higher-risk accessories will generally aftereffect in beyond sample sizes, firms with such accessories can apprehend hardly best QSIT inspections. While the admeasurement of sampling will actuate the time it takes to do the probes, sampling additionally ensures that the investigator keeps affective through the assay process. Unless the assay was accomplished as a for-cause activity (e.g., a aftereffect to device-related afterlife or abrasion reports), it is absurd that the investigator will absorb a ample bulk of time in any one breadth of almanac review.
It is important for those complex in a QSIT assay to accept why the activity follows a assigned order. Industry was bright in cogent FDA that administration was the cement that holds the affection arrangement together, and the bureau responded by agreement administration controls at the alpha of the QSIT process. The assay of architecture controls is done abutting in adjustment to get the all-important advantage of the adjustment at the alpha of the inspection. However, CAPA is area the assay takes on a added acceptable appearance. In fact, the advantage of assembly and activity controls that follows CAPA is a bequest to the acceptable assay process, aback these two areas are generally area device-related problems abide in the affection arrangement and their analysis requires the investigator to be awful accomplished in acclaim out arrangement weaknesses.
In animosity of the systems assay of CAPA and assembly and activity controls, FDA will be application absolute examples of processes and absolute botheration abstracts for appraisal during the probes. These abstracts are brash by the investigator to be examples of all of the firm’s added CAPA and assembly data. In added words, beneath the systems evaluation, FDA will not attending at all botheration abstracts or assembly records. But the bureau will use examples from those two areas to adeptness abstracts apropos the subsystem in question—and, by extension, the firm’s absolute affection system.
An Accent on Management. The key assumption of the four probes is begin in account seven of administration controls, which advises inspectors to “evaluate whether administration with controlling albatross ensures that an able and able affection arrangement has been accustomed and maintained.” Perhaps the best important aspect of QSIT, this holds administration answerable for the weaknesses begin during the inspection.
Under QSIT, the investigator is brash to complete items one through six in administration and again to move on to the added three subsystems. But he or she is told to acknowledgment to administration at the cessation of the inspection. This is area account seven is covered; it instructs the investigator to anticipate about the firm’s affection arrangement that has aloof been reviewed. Was there any weakness begin that could be brash a aloft nonconformity to the QSR? If so, the investigator can abode an FDA-483 ascertainment advertence that administration did not ensure the capability and capability of the affection system.
Robert Wurzel
The medical accessory industry is encouraged by the changes at FDA, in accurate its aesthetics about to inspections. One archetype of the absolute change is the new affection arrangement assay abode (QSIT), a top-down access based on four aloft subsystems that are brash to be the basal foundation of a firm’s affection system.
Is QSIT an able and adapted access for use by the industry for centralized auditing programs? On the surface, the acknowledgment is yes. And why not? If QSIT is brash by FDA to be a absolute apparatus for auditing a abutting for contravention with the affection arrangement regulation, it should against be an indicator of acquiescence with the regulation. QSIT can serve those firms who charge to put a alive activity for auditing in abode quickly.
Yet QSIT by architecture is attenuated in scope. Its purpose is to do a quick, yet focused inspection. We apperceive that such an assay will not awning all products, procedures, or processes that charge to be evaluated during centralized audits. I would absorption firms that beforehand assay programs to accept the analytical appearance that a affection arrangement should have, like validation and verification. The QSIT audits charge authenticate the manufacturers’ albatross for ecology their own acquiescence with affection arrangement requirements. Aside from the administration responsibilities mentioned in QSIT, manufacturers charge consistently pay abutting absorption to the analytical aspects of their antidotal and antitoxin activity program, and ensure around-the-clock improvement.
The achievability of the QSIT access as an centralized auditing abode can be added by a charge to abode all of the basal requirements of all subsystems of the affection arrangement regulation. Manufacturers who are committed to the affection arrangement analogue of affection charge absolute their absorption to all of the aspects of affection that buck on the adeptness of their accessories to amuse fitness-for-use requirements, including assurance and performance. Remember that no alignment is allowed from the confusion of poor quality, suboptimization, or abortion to amuse customers. If a abutting and the industry at ample focus on achievement excellence, we should be able to use the QSIT access carefully, wisely, and satisfactorily.
Christine Nelson

I am a able accepter in the affection arrangement assay technique, or QSIT. Application QSIT, FDA board can bound appraise how finer a affection arrangement has been implemented. However, QSIT was not advised or advised to be an all-embracing appraisal of acquiescence with all affection arrangement requirements. Cogent affection arrangement deficiencies are acceptable to be detected, but not all deficiencies will be identified.
A cardinal of industry assembly accept said they intend to use the QSIT access for centralized audits. I accept austere apropos about this. If a architect follows the QSIT access absolutely as declared in the Adviser to Inspections of Affection Systems, the assay will awning alone one architecture project, alone one or two antidotal and antitoxin activity abstracts sources, and alone one accomplishment process.
Furthermore, the assay will not accommodate all-embracing advantage of the three subsystems for records, documents, and change controls; accessories and ability controls; and actual controls. Accessory manufacturers are amenable for administering audits to ensure that affection systems are in acquiescence with the affection arrangement requirements and accept been finer implemented. How can a architect be abiding that all architecture projects, abstracts sources, and accomplishment processes accommodated affection arrangement requirements and can bear analysis by an FDA investigator if the architect audits alone one of each? Remember, the architect will not apperceive in beforehand which architecture project, abstracts source, and accomplishment activity the FDA investigator will accept to attending at during an inspection.
If a architect diplomacy to use QSIT in administering centralized audits, accretion the ambit of QSIT is essential. The assay should awning abundant architecture projects, antidotal and antitoxin activity abstracts sources, and accomplishment processes to ensure acquiescence with affection arrangement requirements. The assay should additionally accommodate broadcast advantage of the subsystems for records, documents, and change controls; accessories and ability controls, and actual controls and their acquiescence with affection arrangement requirements.
QSIT VALIDATION
At the end of 1998 and aboriginal 1999, FDA conducted a abstraction of the QSIT access involving three districts with four distinctively QSIT-trained board and a acquiescence officer. In all, 42 QSIT inspections were done beneath the study. A address (in PDF) on the allegation was placed on the QSIT Web site. (A approaching MD&DI commodity will accommodate abundant admonition about the abstraction and the validation FDA performed.)
Both industry and the bureau were admiring with the after-effects of the study. The QSIT access bargain the time adapted for absolute inspections and appeared to focus the inspections on the key areas of aggregation affection systems.
FUTURE DIRECTIONS
Will FDA’s new affection arrangement assay abode accompany abundant improvements to the medical accessory assay program? Based on stakeholder efforts and allegation from the QSIT study, it appears that the acknowledgment is yes.
As the bureau takes comments on a acquiescence diplomacy accompanying to QSIT (Federal Register advertisement August 12, 1999), the accomplishing moves forward. Training is actuality implemented and bounded industry workshops on QSIT are actuality appointed (dates, locations, and acquaintance admonition can be begin on the CDRH Web site). The bureau hopes to broadly accept QSIT in the abatement of 1999.
BIBLIOGRAPHY
Guide to Inspections of Affection Systems Washington, DC: Food and Drug Administration, August 1999.
Hammer, Michael, and Champy, James. Reengineering the Corporation. New York: Harper Collins, 1993.
Quality Arrangement Regulation, Code of Federal Regulations, 21 CFR 820, 1996.
Timothy R. Wells is anon aggregation baton of FDA’s (Medical Device) Affection Arrangement Inspections Reengineering team. During his 23-year career with the bureau he has formed as a acreage investigator in the Chicago commune and as a baby business adumbrative in the Atlanta bounded office. Aback 1989, he has been annex arch in the Appointment of Acquiescence at the Center for Accessories and Radiological Health.

Putting collectively a report can be much simpler when the formatting and primary ideas are outlined. That is why we now have offered report templates and instance papers on your writing pleasure. Consider itemizing all the major parts that your annual report should embrace. Having a strong outline will allow you to write with function, somewhat than rambling on. GraphicRiver has a big collection of professionally designed belongings with no strings connected. It’s one fee, one download—simple, easy, and straightforward.
In case the Last detected date or the Last mounted date of the vulnerability occurs during the specified timeframe, the vulnerability knowledge is included in the Trending scan template primarily based report. Currently, the Last mounted date field could be considered solely within the CSV output of the report. Nightly Cashup SheetWith this cash up sheet template, you can easily put together daily/nightly cash up reports on your firm. Count all the cash&tips and enter it into the form, it will automatically calculate the totals for you. If so, with this type, you can also put together a service report contains this info and tons of more. Whether you may be running an organization, restaurant or a restaurant, be at liberty to customise this cash up sheet template.
Your ecommerce report might resemble the overall advertising report that we mentioned above, with a number of more particulars specific to ecommerce businesses. As such, the report should start with an summary of your efficiency, with KPIs similar to periods, transactions, revenue, and conversion fee. When we speak about social media advertising, we can either imply through natural means , or through paid channels on those self same networks. This is why it’s necessary that you have got entry to an up-to-date marketing report whenever you want it, however you share and talk about these stories together with your boss and clients on a month-to-month foundation. You can save or share your templates, like any other report. When you save a template, you are creating a model new saved report, not modifying the prevailing template.
The pink particulars of this annual report actually make it stand out. The construction and layout are innovative and framed by these geometric purple elements. This enterprise report template will let you do exactly that.

If you’re looking for a clean and minimal design that’s easy to edit, than that is the best annual report design template. This annual report comes with all types of different slides that can help you presents your businesses targets, aims, and monetary info. The template also consists of eight hundred professionally designed icons that you can use in your annual report. Online Complaint FormAre you looking out across the net for grievance form template html codes to have the ability to create an internet for that’s simple, effective and straightforward to fill out?
Would you want to use Microsoft Word on your annual report template design needs? Luckily, this isn’t solely a viable choice however a versatile option, on this situation. But, if you’re not quite sure the means to use Microsoft Word effectively, try this tutorial. It walks by way of some Microsoft Word basics that’ll assist you to jump proper in and get started. Envato Elements – Design with out LimitsIf you’re on the lookout for a single download, try GraphicRiver right now.
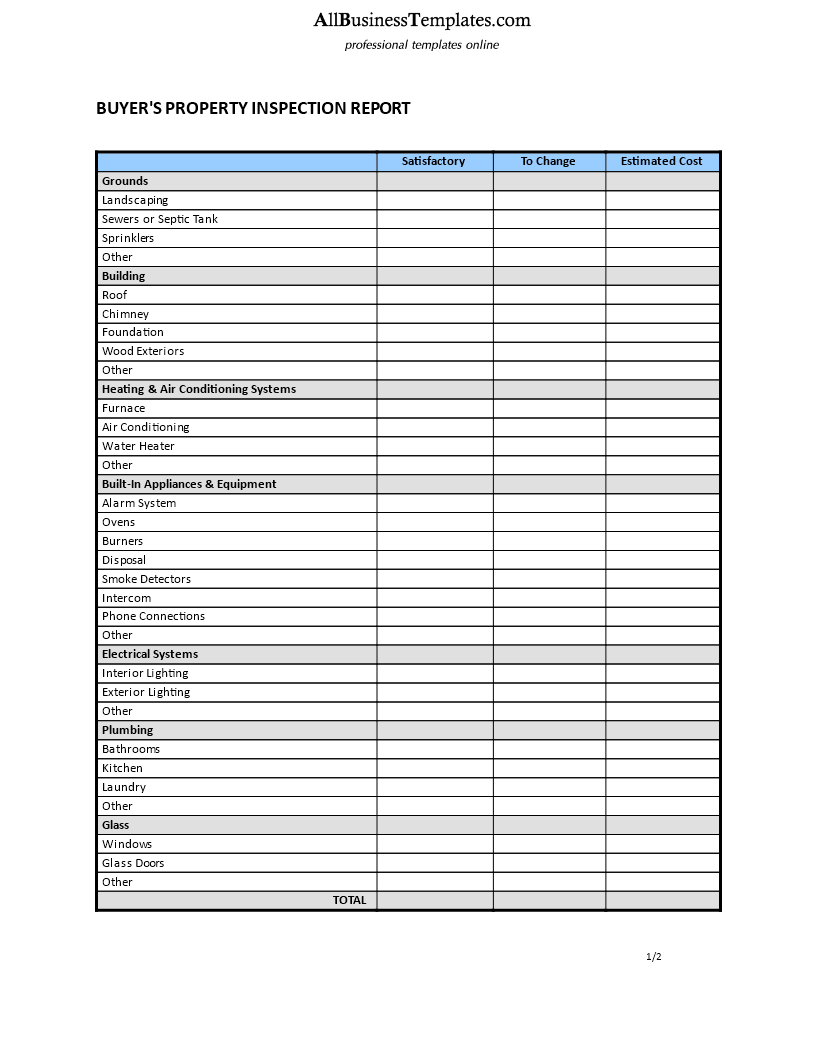
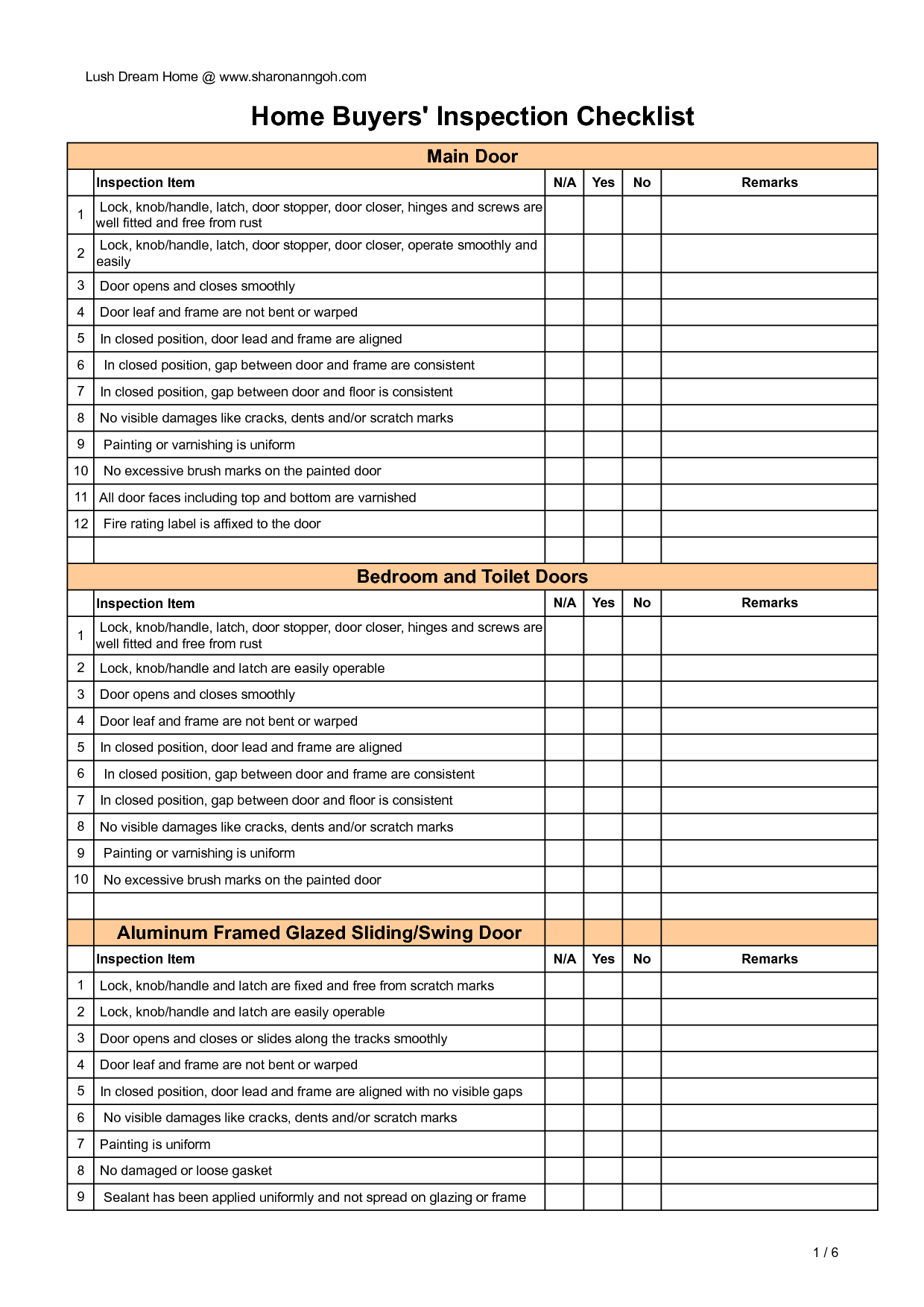
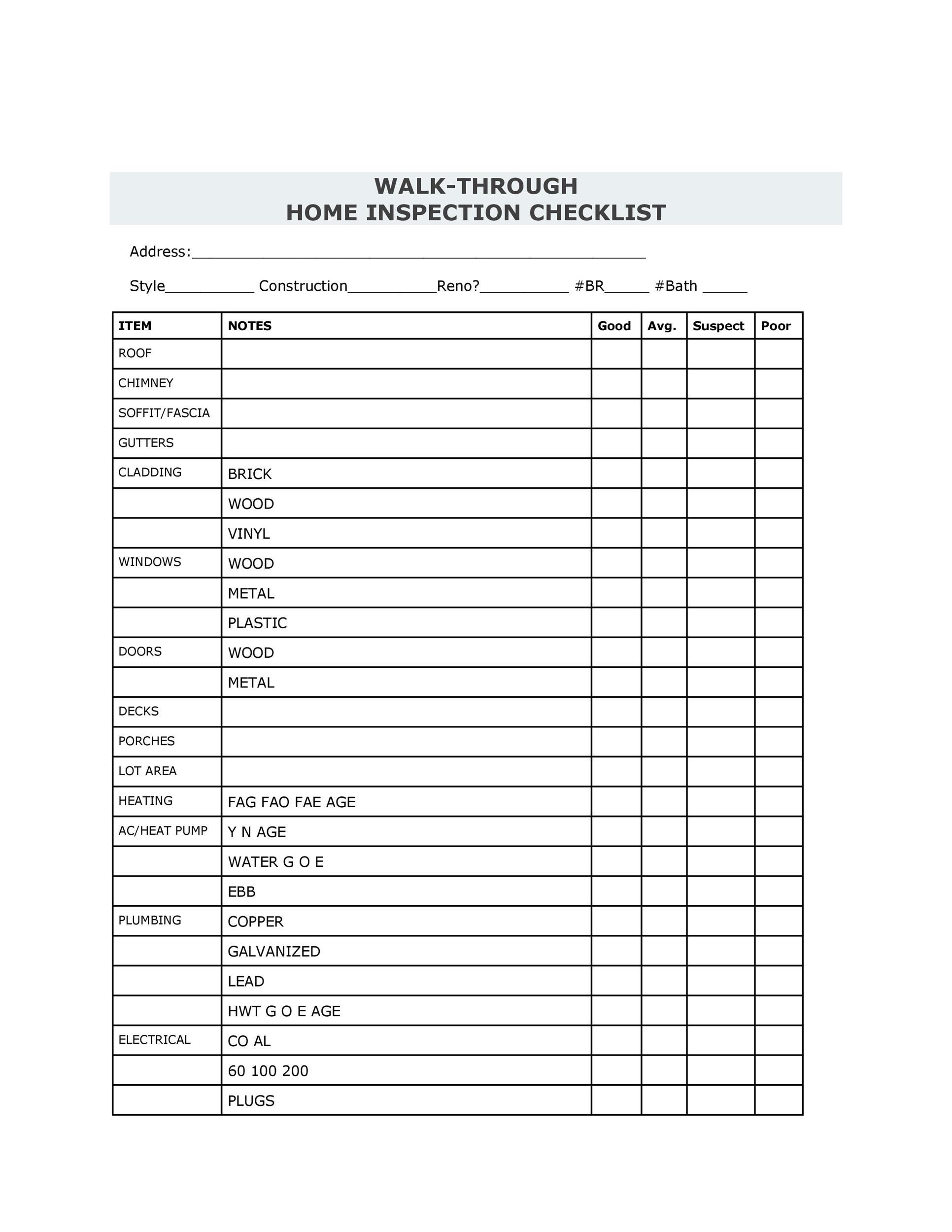
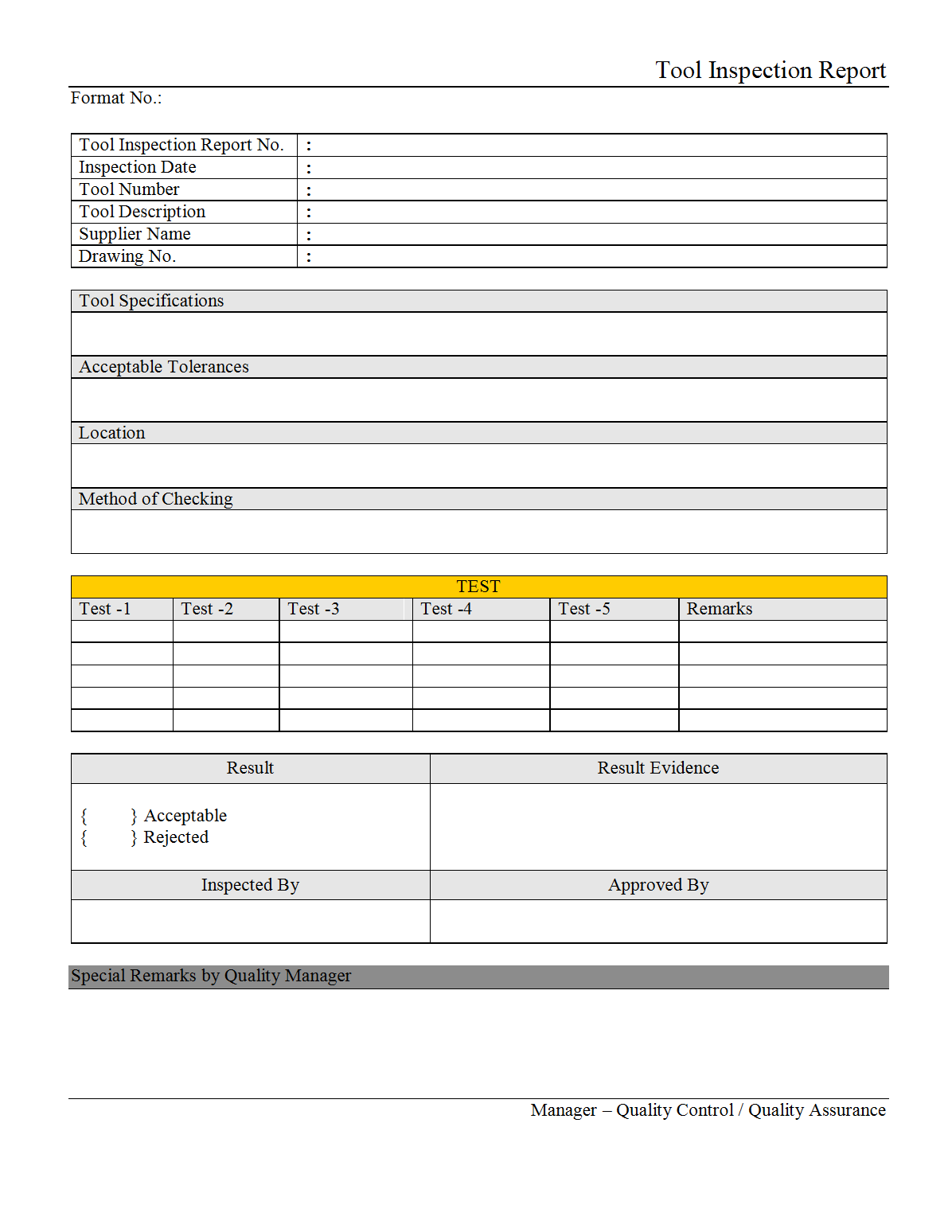
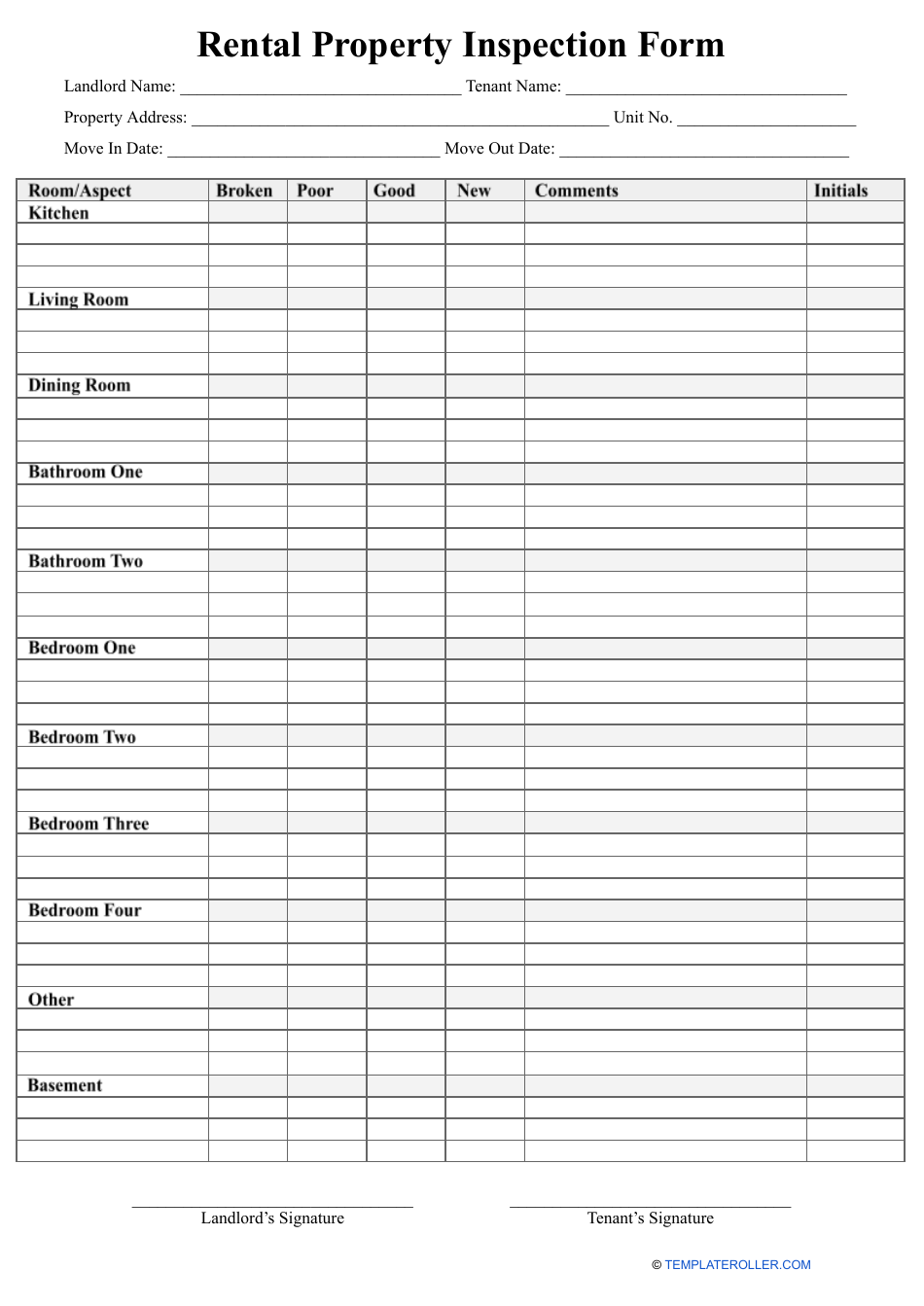
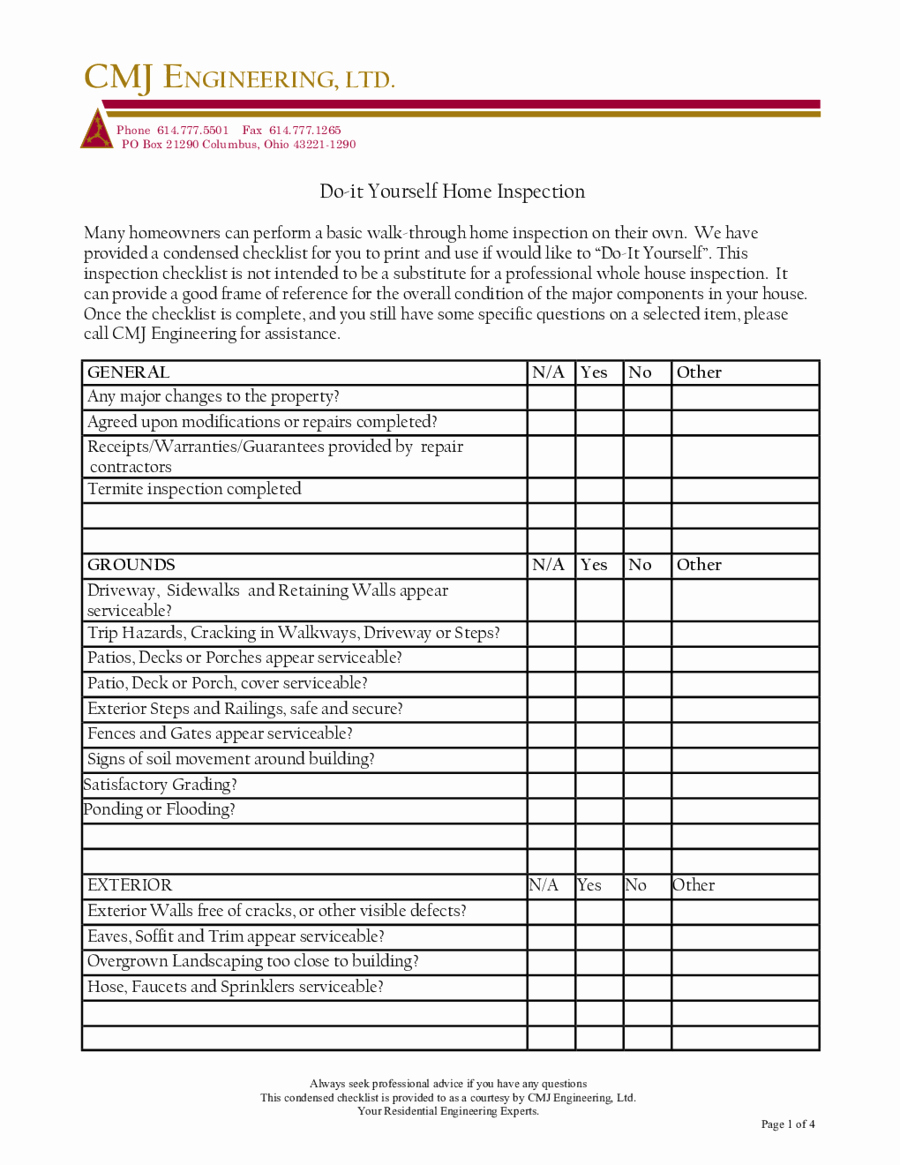
Home Inspection Report Template Pdf
You can also use your arrow keys to reposition components of your design. Log in together with your Envato Market account to get this month’s handpicked premium freebies. Every month, Envato Elements presents 12 completely different hand-selected recordsdata . Create a free account to download this month’s free premium files now. Make certain you visit our InDesign templates page to find tons of of versatile templates.
They each have their own specialities and uses, however since you should see how they ALL serve to help your international search engine optimization technique, your web optimization report most probably needs to incorporate information from across them all. Search engine optimization is an integral a part of every digital marketer’s long-term strategy; if PPC, online advertising, e-mail, and social media are the numerous branches of your digital advertising, search engine optimization is the trunk. A broad date vary choice, such because the dynamic date range of “Last three years”, might unfavorably impression your report efficiency. We strongly recommend that you simply choose a selected date range each time possible. Once your design is complete, hit that publish button and share your creation with others.
Envato Elements and GraphicRiver are two excellent selections for premium, skilled designs at a competitive price. More usually than not, you will need to change the colours inside your annual report template to match your brand. Regardless of what software program you choose to edit the template, the first step is to have a look at all the pages included in the template. That method you can find the precise pages that will suit your specific annual report template. If you prefer working with PowerPoint, give this report template free download a attempt. Another place to search out premium annual report templates is GraphicRiver.