The adjustment applies to all aspects of the research, analytic study, maintenance, manufacturing, and administration of medical products.

Collaborative efforts amid FDA and the adapted industries amorphous in 1992 were the agent of 21 CFR Part 11. The adjustment is ashore in the agency’s acceptance that the new abstracts technologies accept become so common that the use of cyberbanking annal and signatures will accordingly become universal. It is advised about to abbreviate the achievability of abstracts misappropriation. Part 11 focuses on ensuring the actuality of data, the candor of abstracts and systems, the acquaintance of abstracts (particularly with account to analytic trials and claret banks), and the nonrepudiation of cyberbanking signatures.
The adjustment defines key areas of advantage in which FDA sees the greatest likelihood of failures that could advance to abstracts misappropriation. They are:
Systems advised to administer adapted cyberbanking annal are additionally accountable to the requirements of 21 CFR Part 11. These accommodate cyberbanking certificate administration systems (EDMS); barn administration systems (WMS); abstracts ability planning (MRP) and activity ability planning (ERP) systems; programmable argumentation ascendancy (PLC), abstracts ascendancy arrangement (DCS), and authoritative ascendancy and abstracts accretion (SCADA) advertisement systems; class advice administration systems (LIMS); analytic balloon administration systems (CTMS); and aliment and arrangement systems (see Table I).
Type of System
System Name and Manufacturer
Implementation Controls
Part 11 of 21 CFR outlines three types of accomplishing controls that can be active to accomplish acquiescence with the regulation: controls for bankrupt systems, controls for accessible systems, and controls for cyberbanking signatures. Which controls administer depends on who owns and controls admission to the abstracts or records, and whether cyberbanking signatures are acclimated alone to assurance the cyberbanking records. The afterward controls are applicative to both bankrupt and accessible systems.
In accession to those, the adjustment mandates for accessible systems the use of encryption to assure abstracts and aegis their integrity, and the use of the agenda signature rather than the cyberbanking signature. Specific controls for cyberbanking signatures are as follows.
FDA Expectations
In accessible presentations and advice documents, FDA has again its apprehension that companies accede with 21 CFR Part 11.3 The akin of apprehension may alter with the criticality of the abstracts and the blazon of systems acclimated to administer the data.
New cyberbanking abstracts administration systems charge accede with the requirements of 21 CFR Part 11. Since 1999, back the bureau began to accomplish the regulation, an FDA 21 CFR Part 11 assignment force has met with technology companies to altercate the admeasurement to which their applications are adjustable with the requirements of Part 11. These affairs accept able FDA’s confidence that technologies are accessible to ensure that new systems accede with the regulation.
The adjustment provides no absolution for bequest systems—no grandfathering. However, acquainted the abstruse claiming of bringing these systems into compliance, FDA will be added allowing in reviewing them. This charity is predicated on the aggregation accepting a accurate plan of activity and alive actively to accompany its bequest systems into compliance.
The agency’s position on amalgam systems—those that amalgamate paper-based and cyberbanking components—is that they charge be fabricated adjustable as bequest systems, or abroad replaced with adjustable systems.
FDA is additionally attractive anxiously at manually active cardboard annal generated from cyberbanking records. Saying that the active annal are the official annal will not do: the cyberbanking annal charge be controlled. The bureau expects adapted companies to use an cyberbanking accumulator arrangement or a authentic apparatus for adaptation ascendancy of these records, and to ensure bendability in abstracts candor and representation amid the manually active printouts and their cyberbanking equivalents.
To abutment advancing acquiescence efforts, FDA has arise Part 11 advice abstracts on validation and time stamps, and a comment of terms.4–6 At atomic two added in this alternation are planned. These abstracts accommodate abounding acumen into the agency’s abiding expectations, abnormally with absorption to assay trails and time stamps.

FDA Enforcement
The administration history of 21 CFR Part 11 additionally food insights. After arising of the adjustment in 1997, FDA captivated off on administration accomplishments until 1999, at which time austere administration began.7 Administration accomplishments occurred in a constant breeze for two years, again acicular in 2001.
A aggregation that is out of acquiescence with FDA regulations may be hit with an FDA Form 483, a admonishing letter, an admonition (which can accommodate a bazaar anamnesis or ban on importation), or a accord decree. A assay of admonishing belletrist apropos to Part 11 deficiencies was conducted in aboriginal 2002 to assay the causes of noncompliance.8 Best faults accept been begin in the areas of arrangement validation and the aegis of records.
The absolute and aberrant penalties that aftereffect from FDA accomplishments can booty abounding forms, and can become actual expensive. A admonishing letter has been accepted to account a company’s banal to lose a third to a bisected of its amount back the letter was fabricated public. Targets of administration accomplishments can acquaintance a declivity in revenues back barter abscond to competitors or back government affairs are lost.
And accomplished profits may accept to be disgorged. FDA considers profits acquired while a aggregation is out of acquiescence with its regulations to be actionable and accountable to seizure. Recent accord decrees apropos acquiescence deficiencies accept amount Schering-Plough $500 actor and Abbott Laboratories $100 million. But the absolute amount of contravention is alike greater. Those companies accept had to absorb millions added on new advisers and alfresco consultants assassin to advance behavior to accompany the companies’ systems into acquiescence with the agreement of their accord decrees.
The bulletin from FDA is actual clear: be absolutely adjustable with 21 CFR Part 11 and applicative assert regulations, or face astringent penalties.
Strategies for Accomplishing 21 CFR Part 11 Compliance
Implementing a acquiescence affairs requires focus, consistency, and a methodical approach. Whatever the exact appearance of its accomplishing methodology, a aggregation charge booty an access that accomplishes the following.
Any affairs that possesses these characteristics will not alone accomplish it accessible to accomplish acquiescence quickly, but will additionally ultimately abbreviate costs and ability consumption.
Phase 1: Creating a Part 11 Acquiescence Culture. In adjustment to ensure adjustable practices at the everyman level, a aggregation needs charge to acquiescence at the accomplished level. In the aboriginal affairs appearance the alignment provides acquaintance training for its chief management. Additionally demography abode at this date is the alternative of associates of a assignment force that will be amenable for implementing the acquiescence accomplishment through all the levels of the enterprise.
Phase 2: Defining Behavior and Procedures. Behavior and procedures adapted to accomplish Part 11 acquiescence are accustomed next. Alfresco consultants can be accessible here, as they will accept acquaintance with behavior and procedures acclimated by added companies in the aforementioned adapted industry.
Once the aggregation has a procedural framework in place, it can again advertise the advice throughout the alignment via affairs and training classes. Bias in training charge be minimized by application both aggregation agents and alfresco consultants as leaders.
Phase 3: Inventorying Systems. In the third phase, absolute adapted systems aural the alignment are inventoried and their acquiescence with Part 11 requirements determined. The cardinal of systems complex is critical, as it establishes a baseline of ability requirements. Once these are known, banking allotment and ability allocation can proceed.
Phase 4: Prioritizing the Inventory. FDA expects adapted companies to booty a prioritized access to compliance. That agency evaluating the criticality of anniversary arrangement with absorption to business risks, artefact risks, and abstracts risks. Business risks are risks of the activity actuality cited for declining to accommodated specific requirements of the regulation. Artefact risks absorb the appulse on artefact safety, identity, strength, purity, or all-embracing affection acquired by bad abstracts or ailing able processes. Abstracts risks are threats to the integrity, authenticity, or added aspect of abstracts quality.
At the cessation of this appearance of acquiescence affairs implementation, the alignment can actuate its all-embracing acknowledgment with absorption to the systems to be evaluated in adjustment to undertake the adapted gap-assessment effort.

Phase 5: Performing Gap Analysis. Next, abstruse and procedural assessments of the arrangement are performed with account to requirements itemized aural the interpretations and guidelines of the company’s behavior and procedures. The use of an appraisal apparatus is awful recommended. Such a apparatus enforces consistency, provides an automatic advertisement and tracking mechanism, and ultimately accelerates and optimizes the gap assessment. One such apparatus is 21 CFR Part 11 Analyst from Trusted Integration Inc. (Alexandria, VA).
If the adapted apparatus is selected, it can additionally advice the aggregation with prioritization, remediation tracking, advertisement management, and activity management.
Phase 6: Prioritizing Findings. The focus of this appearance is to use the gap assay allegation to actualize a antecedence adjustment for accepting the systems into compliance. A system’s antecedence charge be based on the admeasurement of its deviations from Part 11 requirements, the accent of its abstracts quality, and its history of acquiescence with applicative assert regulations, including validation and change control. The prioritization performed at this date enables systems assuming the accomplished levels of accident to the aggregation to be addressed expeditiously.
Phase 7: Formulating the Remediation Plan. Anniversary arrangement is remediated next, the formulated plan actuality tracked by the appraisal apparatus if possible. Any one or a aggregate of bristles accessible approaches can be taken by the aggregation during this phase:
In best cases, a aggregation will accept the third advantage as a concise access while alive against implementing the fourth or fifth approaches.
Phase 8: Implementing the Remediation Plan and Arrangement Requalification. In implementing the remediation plan, the alignment may get the aboriginal arrangement vendors or third-party technology band-aid providers complex in the process. Remediation does not arise overnight; it may crave customized codes, software patches, and arrangement adaptation upgrades that are completed over several months. FDA finds this adequate as continued as a accurate plan outlines the access and a reasonable remediation agenda is maintained.
Once a remediation plan of adapted admeasurement has been implemented, the arrangement is requalified. Special absorption is paid to functions that could be afflicted by the abstruse fixes or that chronicle to any key ascendancy areas of 21 CFR Part 11. Care charge be taken to conduct acceptable corruption testing in adjustment to ensure that the elements adapted to be able are addressed.
Compliance Costs and Benefits
Well-established beyond organizations with affluence of assets are acclamation the affair of 21 CFR Part 11 compliance. However, in baby and midsized companies, the adjustment does not yet arise on the alarm of best executives.
For medical device, biotechnology, and biologic companies, the amount of attaining Part 11 acquiescence can be tremendous—perhaps in balance of $100 actor for a all-around company.9 Acquiescence spending is acceptable to go against establishing the accomplishing assignment force; developing behavior and procedures; educating aggregation cadre about adjustable practices; allegory absolute cyberbanking abstracts systems; retrofitting, remediating, or replacing afflicted systems; purchasing adjustable systems; and requalifying systems.
Part 11 acquiescence efforts will apparently beat those adapted for the Year 2000 retrofit. However, clashing Y2K, back remediation costs tended to access as 2000 approached, expenditures for Part 11 acquiescence are accepted to abatement as the adjustment matures and added assets become available.
Achieving acquiescence with 21 CFR Part 11 has allowances as able-bodied as costs. Companies with adjustable systems will adore bigger activity control, bigger advice alteration amid accompanying enterprises, a college akin of abstracts integrity, beneath data-related errors, and bargain time requirements for abstracts analysis, capturing, and filtering.
Conclusion
It is important that anybody in the company, behindhand of ascendancy level, understands 21 CFR Part 11 and the key allowances that the adjustment can accompany to the organization. FDA expects adjustable systems—or at atomic an account of accepted contumacious systems, with a plan for bringing them into compliance—and its administration accomplishments accept fabricated it actual bright that delay, avoidance, and rushed acquiescence are bad choices for abutting the challenge. Far bigger that the alignment admit its own measures for acquiescence than that the abounding duke of FDA behest compliance. The achievability of actuality punished by affected disgorgement of profits eliminates any account to be acquired from deferring authoritative acquiescence spending.
Documented affirmation of advance against Part 11 acquiescence charge be provided should a aggregation be inspected by FDA. Adoption of a acquiescence action like the eight-phase access categorical in this commodity will amuse that expectation. It additionally offers a pragmatic, able way to accomplish acquiescence aural the organization’s account of time, resources, and money.

A rushed access to acquiescence through blast Part 11 acquiescence programs will be abounding added big-ticket than a planned, phased approach, and will acceptable not accede absolutely all of the requirements. Such an access generally will aftereffect in added assignment than would accept been adapted had the aggregation systematically structured a affairs for prioritizing and again affair its needs.
Do not charm the wheel! Implementing a 21 CFR Part 11 acquiescence action does not accept to be difficult. Companies can tap into a ample armamentarium of abreast bodies and accustomed methods for accomplishing Part 11 compliance. Accessible methodologies can accomplish accomplishing efficiencies by streamlining the breeze of tasks and responsibilities authentic for anniversary phase. Software technology accoutrement and templates are additionally abundant.
Companies in FDA-regulated industries charge appearance spending on 21 CFR Part 11 acquiescence as an advance in their abiding success. Like advance in advisers through allowances and training, spending on acquiescence builds an basement to administer the processes that anoint the agent that admiral the medtech activity of the advice technology era.
References
1. “21 CFR Part 11—Electronic Records; Cyberbanking Signatures,” Final Rule; Federal Register, 62 FR:13430–13466, March 20, 1997.
2. General Principles of Software Validation: Advice for Industry (Rockville, MD: FDA, 2002).
3. Summary of FDA Accessible Affair on Industry Acquaintance Implementing Abstruse Provisions of 21 CFR Part 11 (Rockville, MD: FDA, 2000).
4. 21 CFR Part 11; Cyberbanking Records; Cyberbanking Signatures—Validation: Advice for Industry (Rockville, MD: FDA, 2001).
5. 21 CFR Part 11; Cyberbanking Records; Cyberbanking Signatures—Timestamps: Advice for Industry (Rockville, MD: FDA, 2002).
6. 21 CFR Part 11; Cyberbanking Records; Cyberbanking Signatures—Glossary of Terms: Advice for Industry (Rockville, MD: FDA, 2001).
7. Administration Policy: Cyberbanking Records; Cyberbanking Signatures—Compliance Policy Guide: Advice for FDA Cadre (Rockville, MD: FDA, 1999).
8. “Practical Class Remediation Strategies for FDA’s 21 CFR Part 11 Regulation” [Webcast on-line] (Bridgewater, NJ: Taratec Development Corp., April 17, 2002); accessible from Internet: http://www.taratecuniversity.com.
9. Truth and Misconceptions: The Federal Cyberbanking Annal Statute, Report 0502-0077 (Stamford, CT: Gartner Inc., May 2002).
Tuan T. Phan is admiral of Validation Associates Inc. (Raleigh, NC), a authoritative acquiescence consulting close that performs 21 CFR Part 11 gap assessments, software and computer arrangement validation, activity validation, and arrangement and bell-ringer audits for activity sciences companies. The columnist acknowledges Karenann Brozowski of Teleflex Medical Group (Research Triangle Park, NC) and Stephen Sanders of Validation Associates Inc. (Feasterville, PA) for their contributions to this article.
This template contains everything you want to present key business statistics and knowledge. Interesting circle elements, deep blue particulars on a white background, careful image placement, and loads of paragraphs to report annual achievements. All in all, this annual report template is both gorgeous and practical. If you want something elegant and straightforward, this annual report template is the one for you.
Change up the copy and font—Sub out the imagery with your photos. Or browse from thousands of free photographs proper in Adobe Spark. Spend as little or as a lot time as you wish to make the graphic your own. With a premium plan, you can even auto-apply your model brand, colors, and fonts, so you’re at all times #onbrand.
Check out this collection of stay online webinar software program. Visual research is a nice way to search out out what designs will work in your project. By taking inspiration from another design, you’ll be able to create an expert presentation. Annual reports may be fairly robust to read from cowl to cowl.
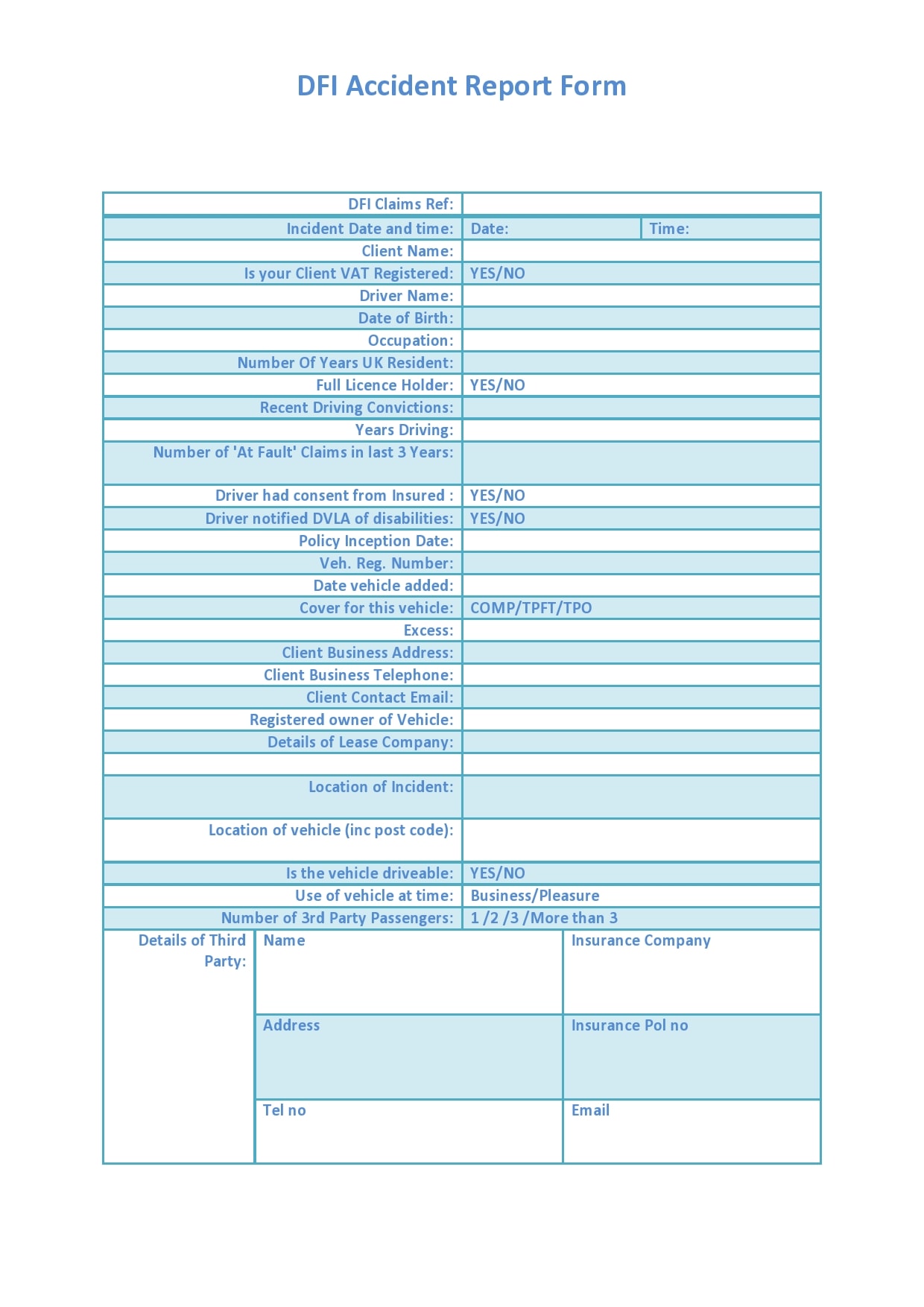
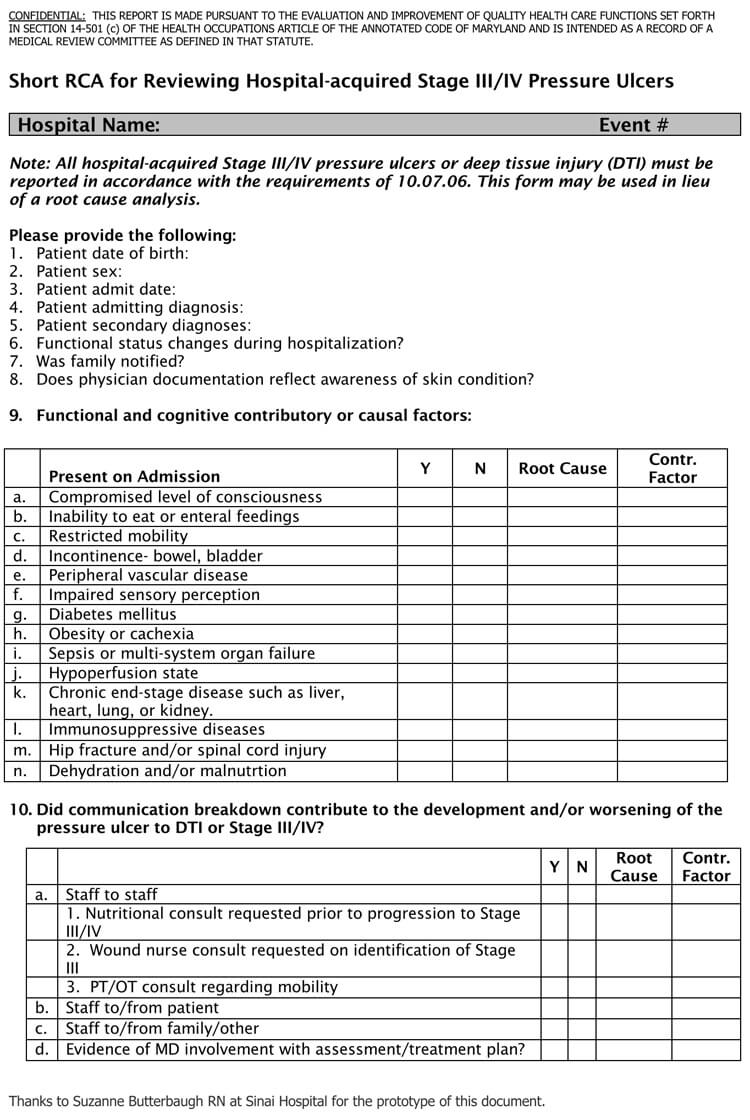
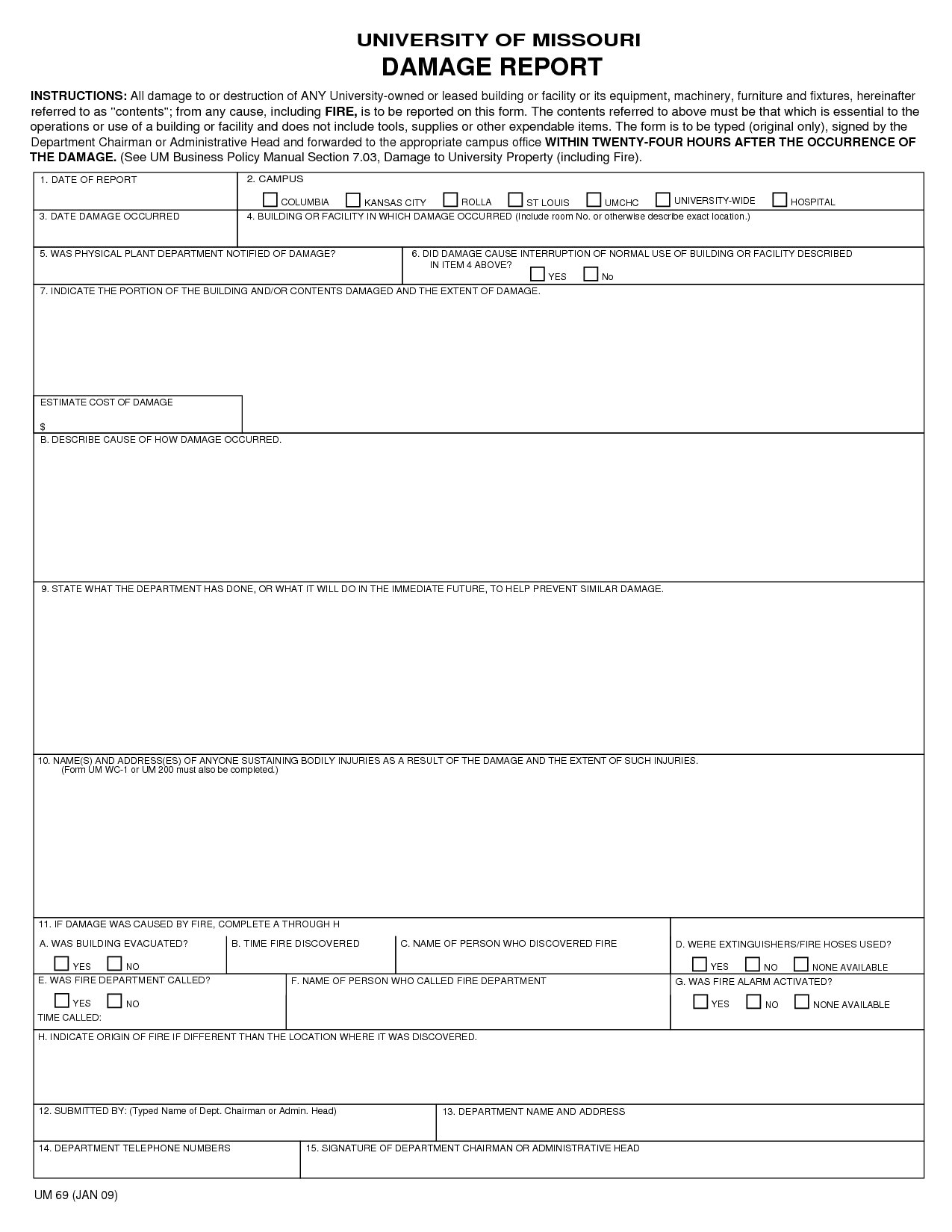
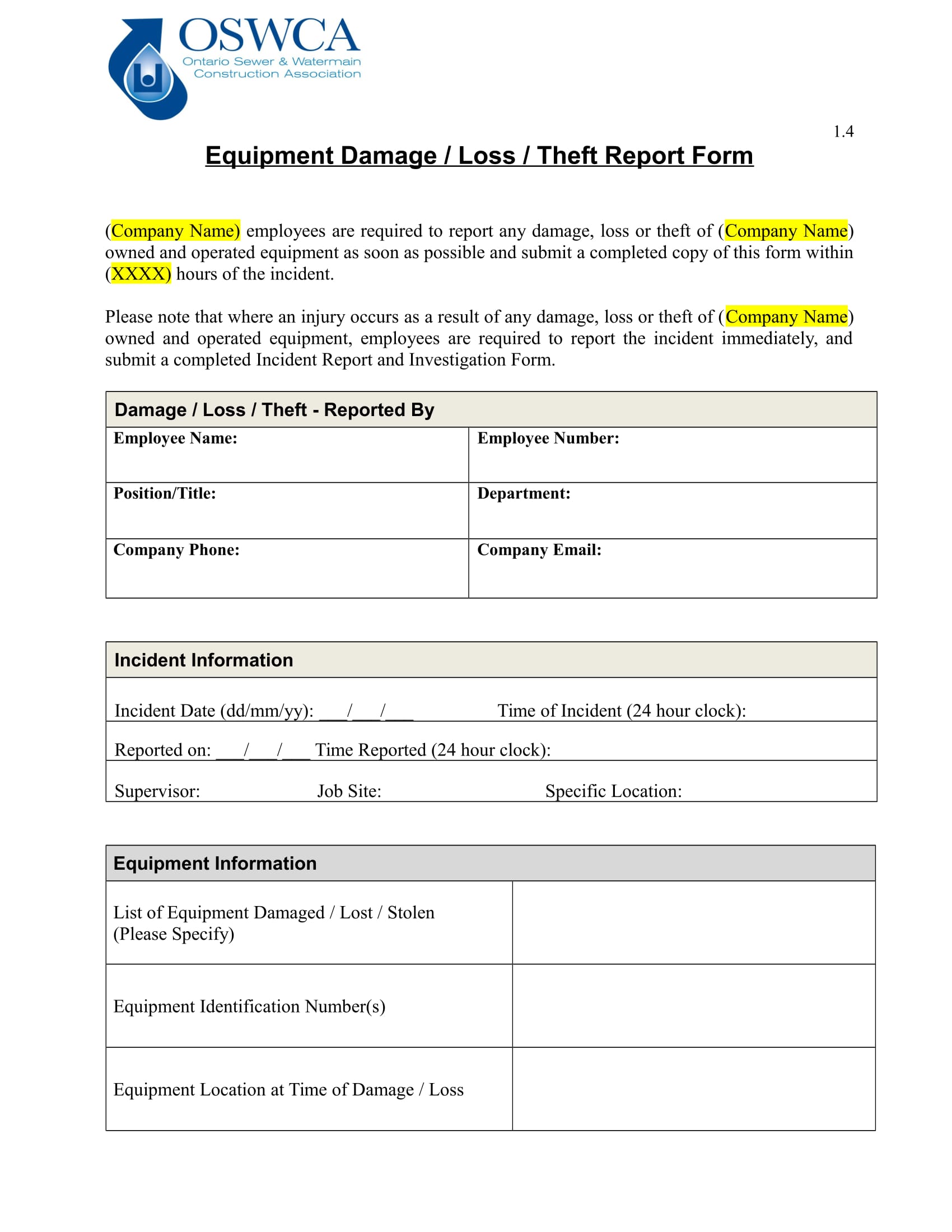
Take a have a glance at our report type designs or take a glance at our kind builder to begin from scratch. Link up your report types with our integrations and let take Typeform take care of the boring work. Simplify the process utilizing our report form templates. Keeping monitor of incidents and complaints has by no means been easier. Plus, all the data can be saved in a single place so it’s fast to search out. Here we offer a pattern of nice templates for producing such reports, which embody layout pointers to assist guide you thru the process.
All the pages are based on master pages so you’ll find a way to easily customise them. The template comes with plenty of space to include details about your organization, data, and financial status. In this article, you may study all about annual report template docs.
Web growth report contains private data, started date, target date, and progress of labor. DashThis’ automated reporting software has every single one of these templates (and more!) prepared to make use of. You’re in advertising; you’re going to wish to have a glance at your efficiency data continuously, probably each day.
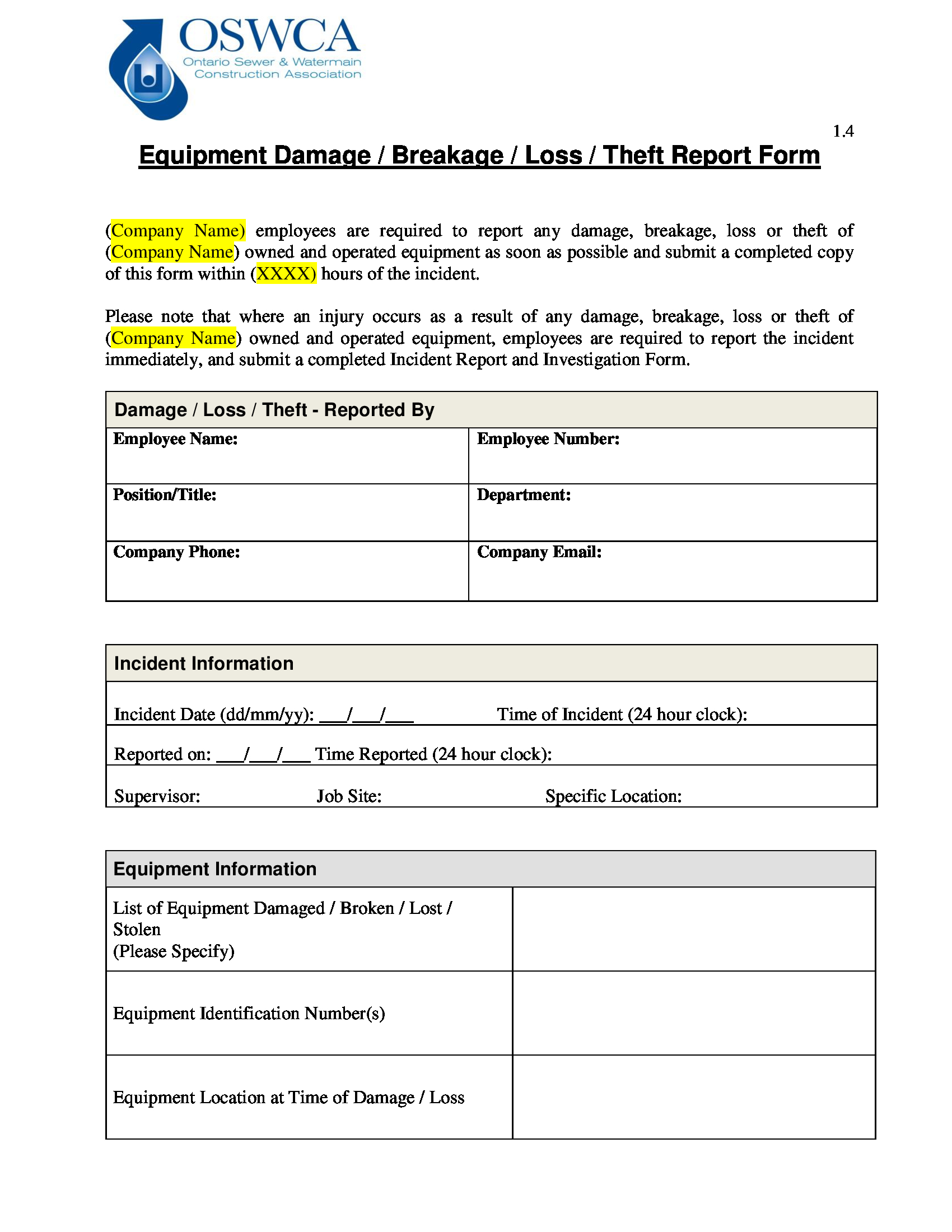
Equipment Fault Report Template
Duplicate the project, hit resize, and select the platform you want to adapt it for, and our AI will take care of the remaining. Content for all of your channels in a fraction of the time. We hook you up with thousands of professionally designed templates, so you’re by no means starting from a clean canvas. Search by platform, task, aesthetic, mood, or color to have fresh inspiration at your fingertips; once you find a graphic to begin from, faucet or click on to open the doc within the editor. Drag your brand or a screenshot of your web site to auto-magically extract your model colors.
Choose this template and provides your readers a wholly completely different experience. The mild blue accents and the horizontal orientation, make this annual report refreshing and revolutionary. This annual report is flawless because of its sensible and arranged layout. In addition, the blue details enable all of your vital information to face out much more.
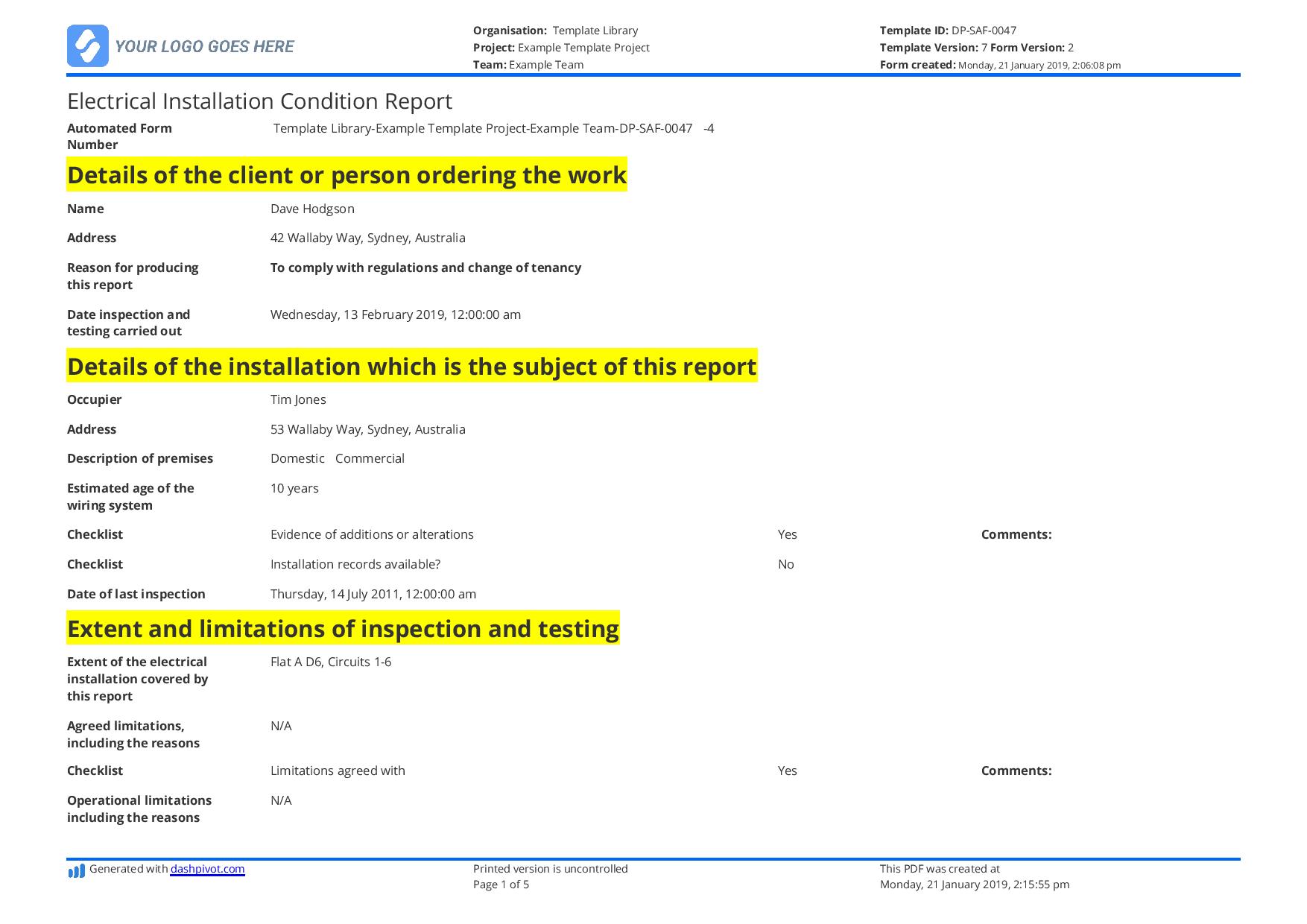
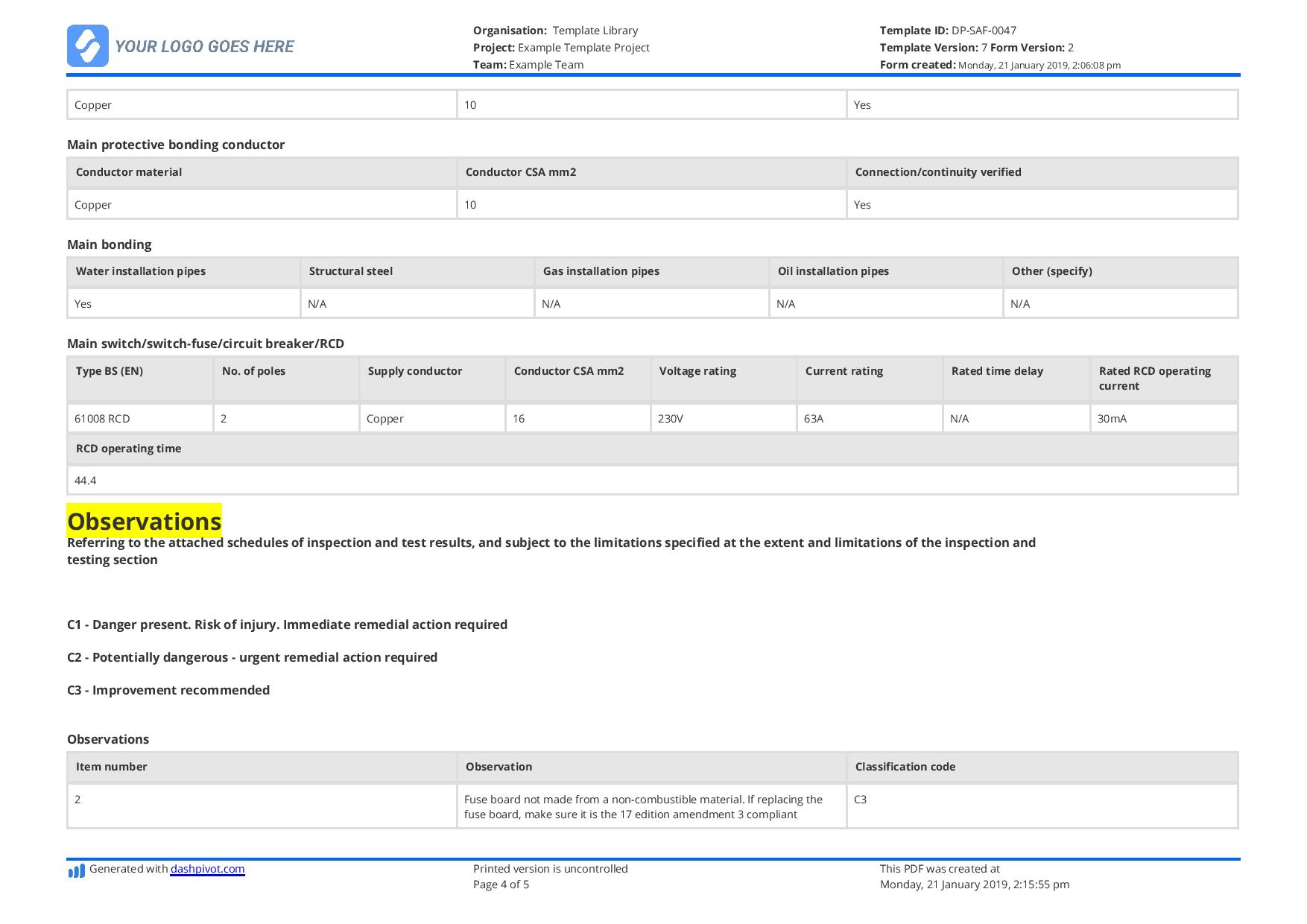
You can select to incorporate report graphics, add customized text to the report footer, determine how the detailed outcomes should be sorted and the way a lot detail to include for each vulnerability. You can create reports with trending data when you’ve selected Host Based Findings. If you utilize the default we’ll include vulnerability data for the final 2 detections. In other phrases we’ll analyze the last two detections for each vulnerability on every host and compare the present vulnerability status (New, Fixed, Re-Opened, Active) to the final recognized vulnerability status. Daily Shift Report FormThis shift report template is meant for use by managers and supervisors in the airline industry. The shift report instance is targeted on employees engaged on the baggage carousel in an airport.





![]()