Clinical Trial Report Template. Typically, the move of your CSR will progress underneath six major headings or sections, not not like these used in a analysis manuscript. Provides a framework for documenting the consent discussion and course of with a possible research participant. Report on key metrics and get real-time visibility into work because it occurs with roll-up reviews, dashboards, and automated workflows built to maintain your group linked and knowledgeable. Guidance documents are also offered to assist you with examine management.
Site staff involved in IP repackaging ought to be a part of an unblinded study team. The following templates present a standard protocol structure and group which can facilitate protocol evaluate by oversight entities. Such a report must be fastidiously prepared and it should summarize the signifies that a research is completed.
The individual composing the store minutes ought to make mention to that such and such topics were talked about and such and such stories had been submitted. We review contracts for sponsored tasks applying regulatory, statutory and organizational data to stability the college’s mission, the sponsor’s objectives, and the investigator’s mental pursuits. These greatest practices apply to all clinical analysis, whether or not the analysis falls beneath IND rules or other regulatory regimes. One of the issues that almost all entrepreneurs rejection like arrival in issue is the utilization of innovation. This template features a proposed construction for a DSMB report as properly as draft language and other steerage.
With a premium plan, you’ll find a approach to even auto-apply your brand model, colors, and fonts, so you’re all the time #onbrand. The manufacturing trial partners and products being trialled are.
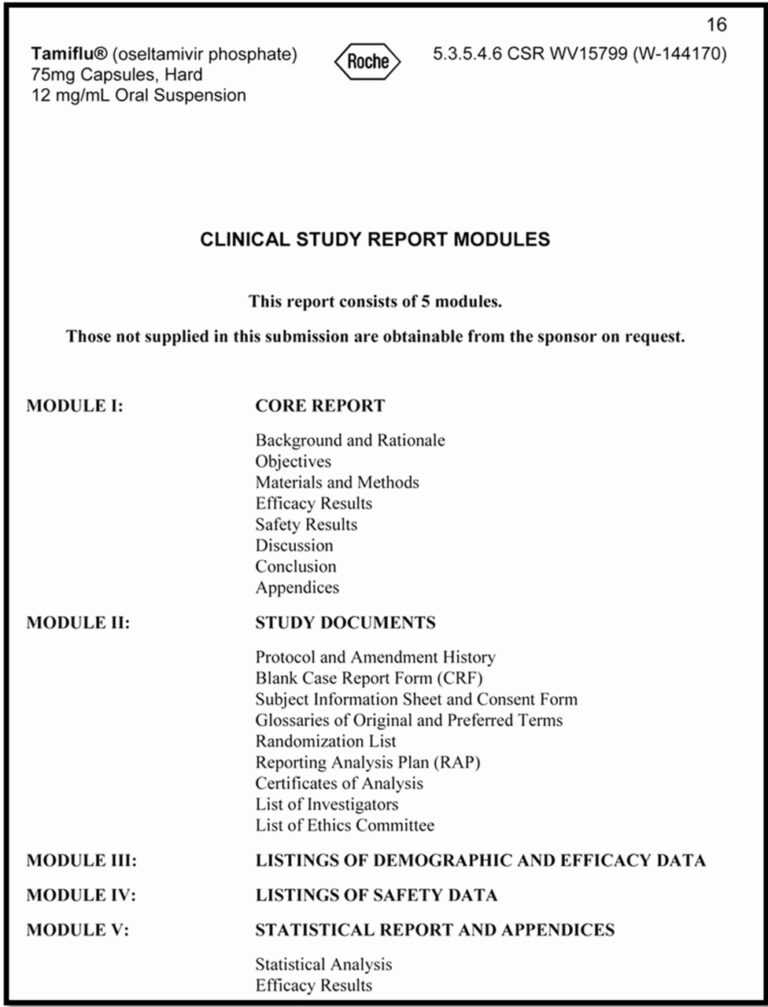
On the entrance finish, even before the background and introduction, the doc will embody a title web page, synopsis, desk of contents, record of abbreviations, ethics statements, and particulars on the study’s administrative construction. The major sections to come after that are highlighted in Figure 2 and summarized in flip beneath.
This template serves to prepare a Site Initiation Meeting to information the content material of the assembly so as to ensure the location is prepared for the correct conduct of the study. Use this log to document IRB submissions, descriptions of submissions, and dates of submissions and approvals. This template data all assigned study-related duties.
Clinical Examine Reports A Hundred And One: Ideas And Tips For The Novice
The final segment of the template has a music for further conversations and for report entries. The individual composing the store minutes should make point out to that such and such subjects have been talked about and such and such stories had been submitted.
A abstract report is typically about 2-3 page-long doc that encompasses the highlights from the trial. Inclusion criteria are the requirements that individuals should meet to enroll in the research.
Scientific Research Tools And Templates
This template is meant for use in monitoring the allotting to and return of examine drug from analysis individuals, after they’ve been given by the Research Pharmacy to the research staff. This guidelines documents and tracks a participant’s eligibility to participate in a study according to the factors specified in the IRB accredited protocol or analysis plan. This template allows the principal investigator and research group to satisfy their duties regarding system accountability record upkeep for significant danger units.

Clinical trials should be conducted with a high commonplace of quality that assures the analysis query is answered in a reliable, legitimate, and unbiased method, and that the rights and welfare of human topics are protected. NIAMS has standardized procedures and supplies templates to ensure compliance and the protection of human topics. When conducting a clinical trial, it is the investigator’s duty to ensure each member of the research staff is skilled on the protocol because it applies to their job function.
This rearranges template withhold – all reports are put away in one spot, and permits to chop off the entry rights to various templates. I noted only a single nonexistence of this methodology – outrage intricacy of the code…

The following templates present a standard protocol structure and group which might facilitate protocol review by oversight entities. Exclusion criteria specify the characteristics that disqualify participants from participating within the analysis.
While there was a variety for the timelines reported, these information present the novice CSR author a primary reference point for the way lengthy the person processes can anticipate to take with experienced medical writers. Fortunately, whereas TLFs are being crafted, a number of other “Writing and Document Review” duties from Table 1 could be performed simultaneously.
The presence of tutorial workout routines permits the people who’ve never utilized any comparable programming to create reports to begin making stories the least demanding and quickest method. In the style of PDF Generator, reviews can be made in minutes.
The additional alternatives with the plan evaluation draw attention to checks the grant related place of the concord to guarantee that you have entered improve charges that are affordable. It moreover reveals a unquestionable earn incite the original investment research simply as other important financial pointers and markers once fairly priced earnings projections. Along these lines, the template causes you kind out your contemplations, and it goes more or less as a find to creation the pretentiousness towards arranging.
Please let me know any other websites/links that present free or inexpensive lectures on clinical Research. I earlier launched it to you due to your prolific curiosity in well being care info and resource sharing….
Data and Safety Monitoring Plan Template and Guidelines and DSMP Checklist have been developed to help investigators in preparation of a sound information and safety monitoring plan. All scientific trials require study-specific monitoring procedures to make sure participant safety and data integrity.
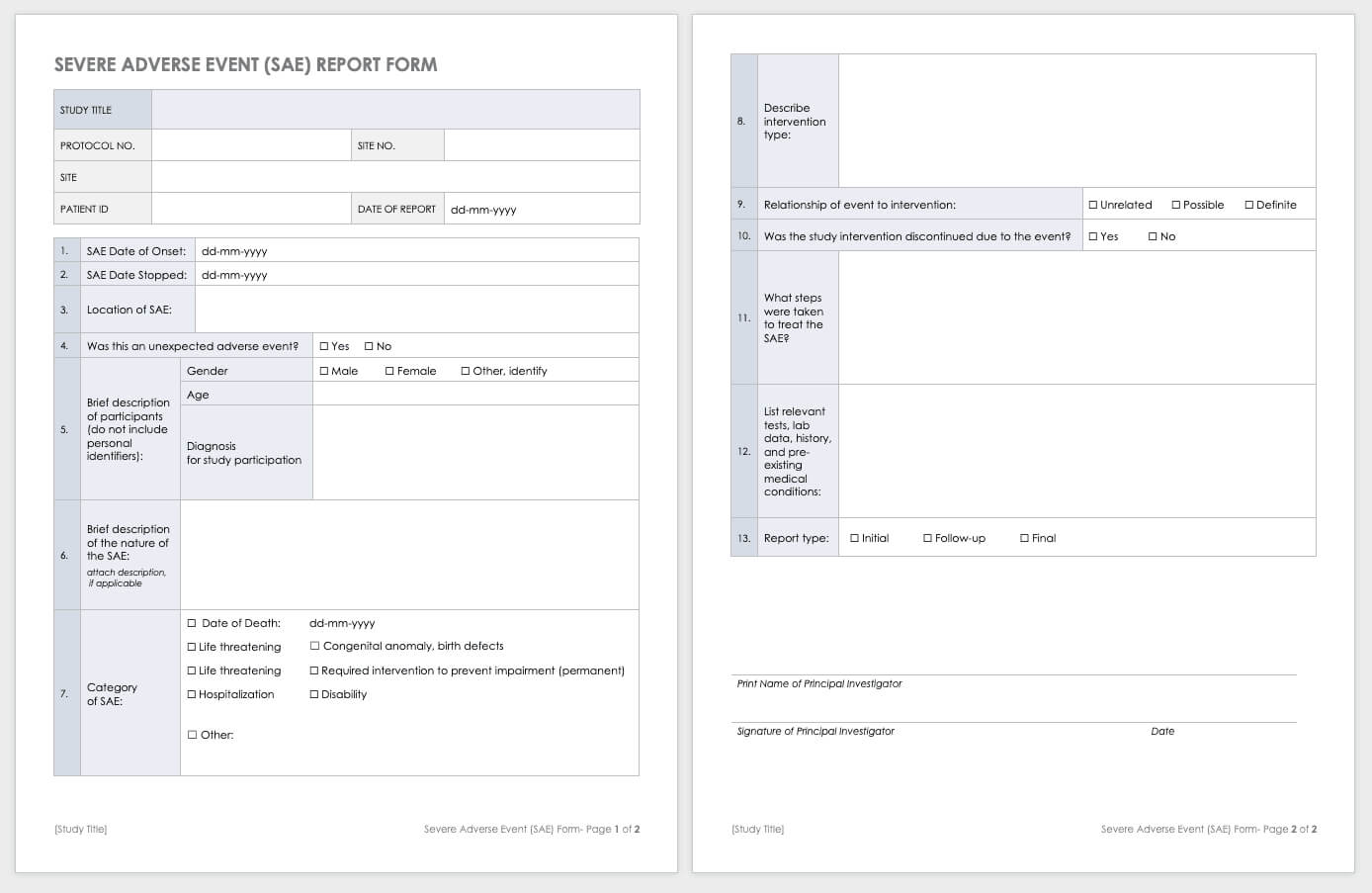
The date on which the report was generated can also be included in the report. So creating a shift report by yourself is not an enormous chore should you discover the right sample template and editing process.

SharePoint manages and shops documents, and it enables a number of customers to entry the documents via their own site or a standardized Microsoft website. A subscription to Microsoft Office 365’s SharePoint doesn’t require a server, however customization options are limited; the flexible authentication and authorization techniques are in-built. Enterprise See how you can align international teams, construct and scale business-driven solutions, and allow IT to handle danger and maintain compliance on the platform for dynamic work.

A first rate coarse psychotherapy Clinical Trial Report Template is bound in structure back it is made to incite you past bettering comprehend your self just as your adversaries and the way they work. You ought to select a template that is moderately basic in construction and pure to accumulate it. For instance, a two section report accomplished in a textual content dimension big passable to be successfully decipherable ought to be agreeable so that you can put terrifically significant data and in the method of to peruse and comprehend the outcomes.

One of the world’s largest impartial biotechnology corporations, Amgen was established in Thousand Oaks, California, in 1980. Amgen’s Thousand Oaks workers in 2017 numbered 5,125 (7.5% of total metropolis employment) and included lots of of scientists, making Amgen the biggest employer in Ventura County.

Biological Sample Handling Log31 KB This template could be used to document the location and identification of the retained organic samples, in addition to the accountability of the samples collected from trial members and shipped to sponsor. It must be used by the unblinded group to document the IP repackaging and relabelling process, and serves to guarantee that the process is performed in accordance to GMP guidelines.
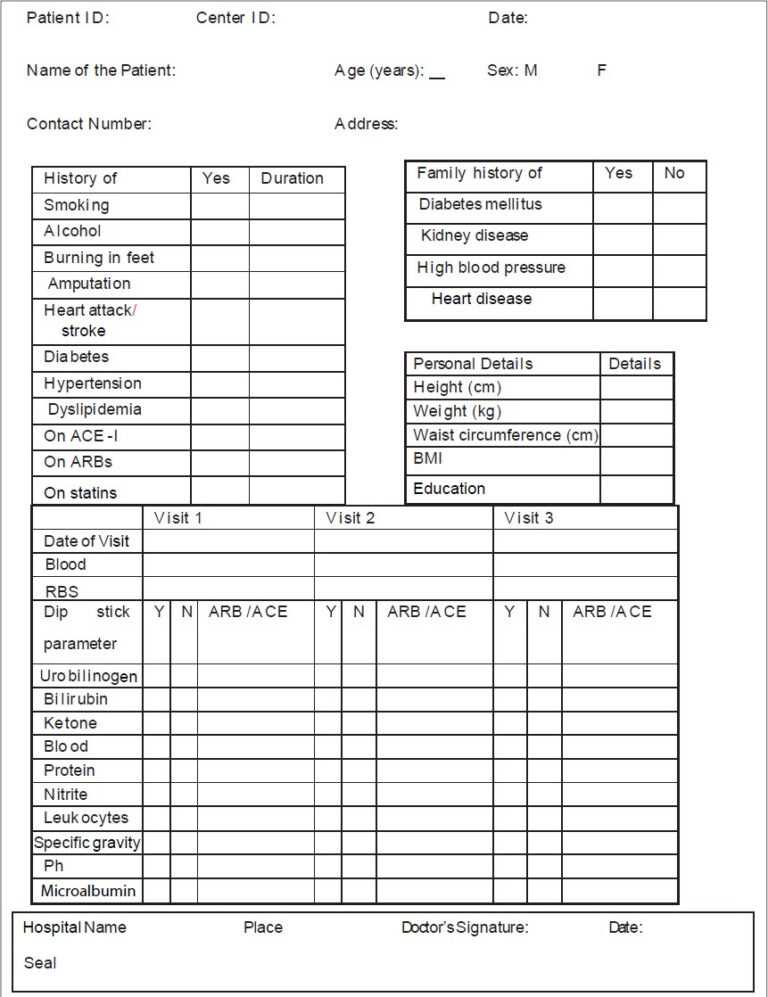
Tools and templates for developing a guide of working procedures to facilitate consistency throughout examine members and website. One of the core components that it could be best to edit in your annual report template is the text.

This web site is using a security service to guard itself from on-line assaults. The action you just carried out triggered the security resolution. There are several actions that would set off this block together with submitting a certain word or phrase, a SQL command or malformed information.

May be used to doc and report unanticipated problems to the study sponsor and the IRB. Used to list all examine personnel and their particular duties, signatures, and dates of obligation in the course of the conduct of a clinical research examine. Provides an at-a-glance reference for together with important documents for scientific research in a Regulatory Binder.

This form, used in those research where the study article is blinded, tracks when a participant’s research article is unblinded. This template lists all the protocol deviations from a specific examine. The template may be used to submit accrued deviations to the IRB at the time of a continuing review for a examine.

The forms under are commonly utilized in medical studies and may be customized to fulfill the needs of the particular scientific examine. Investigators might must create extra types to capture all knowledge components required within the medical study. Templates for investigators, examine staff, data managers, research statisticians and others involved in submitting periodic stories to NIAMS-appointed, unbiased monitoring our bodies (e.g., an independent Data and Safety Monitoring Board or Safety Officer).

Initial Proposal Concept Form – This type ought to be used to advocate for an initiative by the Division of Geriatrics and Clinical Gerontology for a scientific trial or trials that exceed $2 million in direct prices in any year of funding. These finest practices apply to all scientific research, whether or not or not the research falls beneath IND rules or different regulatory regimes.
The Office of Contract Administration can be a part of the Office of Finance – Sponsored Programs. The MW develops a first draft, which can bear an inside staff evaluation and QC earlier than being sent to the consumer for evaluation. However, there are instances when it takes more than three drafts to supply a ultimate report.

Each participant in a medical trial should have an adverse occasion log that tracks any antagonistic occasions via the length of the study. Check out format of production plan in excel complete scheduling of your project towards specific scheduleThese templates are prepared to assist each manufacturing and repair offering corporations from initiation until execution of the event and process. A daily production report is a crucial tool for administration reporting in the manufacturing unit.
A few functions require the placing away of Clinical Trial Report Template in a database. This rearranges template maintain – all reports are put away in a single spot, and permits to surgically remove the entry rights to various templates.
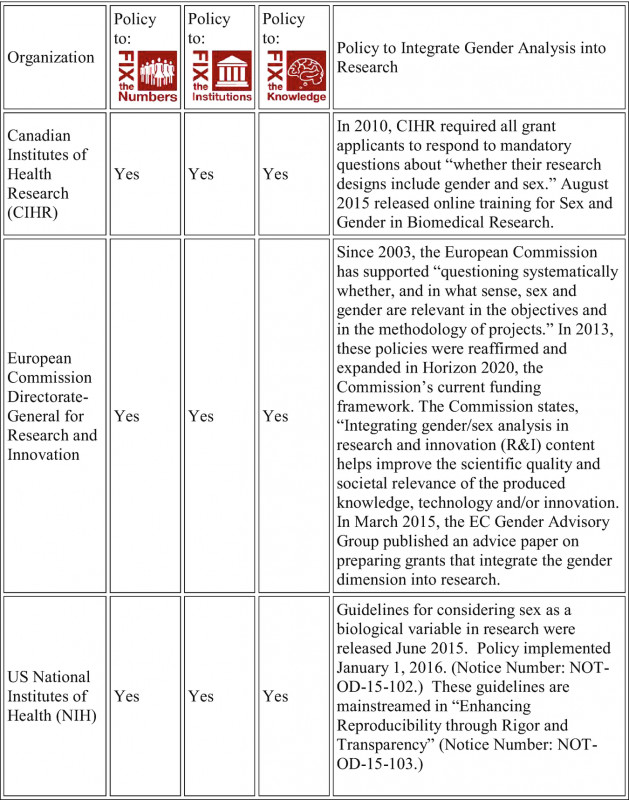
For particular causes, such template have provided right of entry to electronic accretion minutes Clinical Trial Report Template. A addition minutes template comprises of a way the place the herald of the collection and the date might be composed.
Check that every one essential documents are complete, accurate, legible and current. Site workers involved in IP administration should be delegated by the Principal Investigator on the Signature Sheet.

May be completed by NCCIH investigators for research utilizing an investigational product; supplies a comprehensive listing of product disposition on a subject/participant degree. Assists in compliance with the protocol and Good Clinical Practice. Properly documenting any drugs that individuals are taking is imperative to understanding the reactions occurring of their bodies, in addition to what could spur opposed and severe antagonistic occasions during the research.



