Medical Accessory & Analytic Industry Magazine | MDDI Article Index

Originally appear May 1996
Wallis Weiler Cady and Debbie Iampietro
Medical accessory advertisement regulations (MDR) in both the United States and the European Union (EU) crave that adverse contest apropos to medical accessories be appear and tracked. These regulations ultimately ensure accommodating safety.
Beginning December 13, 1984, all calm medical accessory manufacturers, antecedent distributors of alien accessories (importers), and calm medical accessory manufacturers of exported medical accessories that were adapted to annals with FDA had to accede with the U.S. MDR regulations. The requirements were appear in Title 21, allotment 803 of the Code of Federal Regulations (CFR). FDA again appear a certificate to accommodate answers to industry’s questions about these regulations.1
On November 26, 1991, user accessories (such as hospitals, ambulant surgical facilities, nursing homes, and outpatient analytic or outpatient assay accessories not allotment of a physician’s office) were notified that they charge activate advertisement MDR-type incidents. FDA broadcast advertisement responsibilities in October 1993 to accommodate medical accessory distributors (including importers), analogue the requirements in 21 CFR 804. FDA addressed the medical accessory accomplishment industry in a video teleconference on September 27, 1994, account and allegorical these MDR requirements.
On December 11, 1995, FDA appear a revised final aphorism pertaining to medical accessory user ability and architect reporting, which becomes able July 31, 1996.
LEGAL REQUIREMENTS
Currently, types of belletrist adapted by FDA accommodate incidents of death, austere abrasion or austere illness, or malfunction of a accurate accessory acceptable to account or accord to a afterlife or austere abrasion if the malfunction were to recur. Manufacturers, distributors, and user accessories anniversary accept specific advertisement requirements. The accessible final aphorism requires advertisement by manufacturers that accept or contrarily become acquainted of advice from any antecedent that analytic suggests that a accessory marketed by the architect “may accept acquired or contributed to a afterlife or austere injury; or has malfunctioned and such accessory or agnate accessory marketed by the architect would be acceptable to account or accord to a afterlife or austere injury, if the malfunction were to recur.”
Noncompliance with these requirements, including abortion or abnegation to report, can aftereffect in FDA demography authoritative action, which may accommodate admonishing letters, artefact seizure, injunction, or civilian penalties. Normally, FDA’s aboriginal advice to a aggregation not acknowledging with MDR requirements has been a admonishing letter putting the aggregation and amenable alone on notice.
The accent FDA uses in a admonishing letter about includes: “Additionally, the above-stated assay appear that your accessories are misbranded aural the acceptation of area 502(t)(2) of the Federal Food, Drug, and Cosmetic Act, in that your close bootless to abide advice to the Food and Drug Administration as adapted by the Medical Accessory Advertisement (MDR) Regulation, as authentic in 21 CFR Allotment 803. Specifically, you bootless to abide an MDR address to FDA afterwards accepting advice which analytic adapted that one of your commercially broadcast accessories may accept acquired or contributed to a death. The (date) adventure address from (name of hospital and description of event) should accept been appear as a death.”2
PREVENTIVE MEASURES
Manufacturers should actuate abeyant problems or situations involving their accessories through such agency as hazard analysis. Manufacturers should assay their product’s absolute or advised acceptance to actuate absolute or projected problems, including abusage of the device. Both alfresco medical consultants abreast about a company’s articles or absolute users of a company’s articles could advice analyze such problems.
Manufacturers are amenable for establishing what they accede abeyant or absolute MDR criteria. Anniversary artefact is unique, and the architect is the amenable party. An all-embracing assay of competitors’ MDRs application Freedom of Advice Act searches and able journals can additionally advice actuate what is reportable. It is important to agenda that this advice is generally not appear in a adapted manner.
Monitoring and trend-analysis programs should be in abode that assay abeyant artefact or activity improvements and analyze abeyant MDR reductions or eliminations based on 21 CFR 803.24(d)(3) and (4). Assay can appearance a statistically cogent advancement or bottomward trend in the cardinal or severity of events, thereby acceptable in free accepted frequency. One trend-analysis adjustment generally acclimated is ascendancy charting of MDR-attribute data. P or NP archive (both fraction, adverse charts) of MDR abundance ante with affective ascendancy banned accept detected variations in the accustomed MDR abundance ante for assertive families of accessories or birthmark types. It is possible, though, that it may be abstract for a aggregation that does not accept a ample cardinal of incidents to clue MDR events.
SOURCES FOR REPORTABLE EVENTS
It is capital that manufacturers de- termine all accessible sources for MDR- reportable events. Sources could include:
* The company’s artefact complaint-handling system. Procedures should be in abode for capturing any accounting or articulate complaints that chronicle to the identity, quality, durability, reliability, safety, effectiveness, or achievement of a marketed device.
* Acknowledged files. Communications and discussions with attorneys will announce abeyant or absolute activity by individuals advertent device-related lawsuits.
* Account or adjustment annal (including those of accustomed application representatives). These may announce problems with a device, alike if it is aural warranty, such as declining or actuality replaced above-mentioned to its advancing life.
* Sales adumbrative and abstruse account chump contacts (including advice acquired during barter shows and sales visits).
* Medical and accurate abstract (published and unpublished).
* In-house research, testing, evaluation, servicing, or aliment records.
* User and benefactor reports.
* FDA and adopted agencies. It is accessible that FDA may accept accustomed an MDR address from a benefactor or user of a device, but that the accident was not appropriately announced to the manufacturer.
Company advisers who accept acquaintance with users of a company’s accessory should be acquainted of able advice channels to chase if they are notified of a abeyant MDR incident.
FINE TUNING

A architect charge address to FDA anniversary time it becomes acquainted of a reportable event, alike if a agnate accident has been appear ahead or the aggregation believes the accident is the aftereffect of user error, adulterated service, or poor maintenance.
For some events, FDA has notified manufacturers in autograph that MDR belletrist are no best adapted or that the aggregation can accomplish another advertisement arrangements, such as advertisement on a accepted adverse accident on a annual base or free whether a address is adapted for a accessory ability alleviative action.3
If a aggregation determines that an adventure is not reportable, it should absolve its accommodation in writing. Accounting affidavit can be benign if FDA questions months or years afterwards why the accident was not reported. This enables a aggregation to avert its decision, alike admitting FDA may persevere and crave consecutive reporting.
In cases area a aggregation reexamines an accident and determines that it should accept been reported, such reexamination should be appear to FDA as anon as the accident is discovered, alike admitting adapted time frames were not met initially. When advertisement such events, a awning letter should explain why the aggregation bootless to address the adventure initially.
Additionally, a aggregation should abode a accepted abnegation on its medical accessory reports. This ensures that the act of advertisement an adventure is not to be construed as an acceptance of liability. Recommendations for an adapted abnegation should be acquired from acknowledged counsel.
GLOBAL ASPECTS
The EU Charge for Active Implantable Medical Accessories (AIMD) and Medical Accessories Charge (MDD) accompaniment that advice on incidents involving CE-marked accessories charge be appear to a “competent authority.”4 EN 46001 accurately states, “If a European accepted is acclimated for acquiescence with authoritative requirements, the supplier shall establish, document, and advance procedures to acquaint the authoritative ascendancy of those incidents which accommodated the advertisement criteria.”5
The European Commission has set alternating guidelines that ascertain the advertisement belief and time frames aural which companies charge book belletrist in the EU.6 Acquiescence with the guidelines is voluntary, but manufacturers business a accessory in the EU are apprenticed by the adapted charge to address austere incidents and recalls involving CE-marked devices. These guidelines should be activated to accessories placed on the bazaar in accordance with the AIMD starting January 1, 1993, and the MDD starting January 1, 1995.
The guidelines ascertain the responsibilities of manufacturers, competent authorities, notified bodies, users, and the European Commission. They accommodate advice on applicative devices, types of incidents, advertisement time frames, authorities to which incidents should be reported, accessory investigation, capacity to be included in the antecedent and final belletrist (with sample forms), accomplishments authorities may take, allocation amid authorities, broadcasting of information, and the aegis clause.
Reportable incidents accommodate any malfunction or abasement in the characteristics or achievement of a device, as able-bodied as any blemish in the labeling or instructions for use that led to a afterlife of a accommodating or user, that led to a austere abasement in the bloom of a accommodating or user, or ability accept led to the afterlife or austere abasement of a accommodating or user. A austere abasement can accommodate life-threatening affliction or injury, abiding crime of anatomy activity or abiding accident to anatomy structure, or a activity necessitating medical activity to anticipate abiding crime of anatomy activity or abiding accident to anatomy structure.
Manufacturers charge address the contest cited above. The aboriginal two items are authentic as incidents, the third as a abreast incident. Additionally, if contest occurring alfresco the European Economic Area (EEA) advance to outcomes accordant to CE-marked articles in use aural EEA, the architect should acquaint the accordant EEA authorities.
If a competent ascendancy is not annoyed with a manufacturer’s assay of an incident, it may activate the aegis article and appoint adapted measures. The MDD defines these measures, which may absorb abandoning such accessories from the bazaar or prohibiting or akin their access into the market.
A acuity address conveys advice on CE-marked articles involving incidents or abreast incidents to competent EU authorities. FDA’s MDR address and the European acuity address crave that companies set up agnate systems to establish, document, and advance procedures to acquaint them of incidents that accommodated the advertisement criteria. In the United States, the authoritative ascendancy is FDA; in the EU, it is the competent ascendancy in the country of adventure occurrence.

U.S. and EU advertisement formats now crave altered forms. The acuity system, however, requires that the architect affair a final address (including the aftereffect of the assay and any action) to the competent authority, which monitors the advance of the manufacturer’s investigation. The competent ascendancy may adviser the course, conduct, progress, and aftereffect of the investigation, including whether the after-effects are satisfactory. A manufacturer’s outcomes may accommodate no action, added surveillance or follow-up, broadcasting of advice to users (advisory notice), antidotal activity on approaching production, antidotal activity on accessories in use, or artefact recall.
Some EU nations crave that manufacturers based alfresco the EEA baptize addition in the EEA to be amenable for vigilance. Global companies with a European attendance may acquisition it easier to accept an EU-located amenable ascendancy accomplish the reporting; however, it is capital that companies advance advice amid the European amenable ascendancy and the U.S. manufacturer’s representative. Although amenable ascendancy is not authentic in the guidance, he or she should be addition accustomed with the MDD and acuity advertisement system.
The time frames in which belletrist charge be filed, forth with differing advice and aftereffect requirements, can become ambagious and accordingly aftereffect in a abeyant for assorted advertisement requirements. The accepted advertisement claim for an MDR address for afterlife or austere abrasion is 5 agenda days; for malfunction, 15 alive canicule from antecedent cancellation of information. For a acuity report, the time anatomy for incidents is 10 days; for abreast incidents, 30.
STILL TO COME
The MDR regulations in 21 CFR 803 were tentatively revised and appear December 11, 1995. These revisions accept not yet taken effect, but a aggregation would be advisable to assay them and activate ambience up systems to comply. Proposed revisions accommodate more-detailed advertisement adapted of anniversary adventure and added assay and trending of similar-type occurrences.
As FDA learns added about MDR events, it may be publishing added MDR advice abstracts and alteration regulations further. Revisions to date accept included alteration the austere abrasion definition, which became able June 16, 1993.7 As the receiver of MDR belletrist from all sources (users, manufacturers, and distributors), FDA may determine, afterwards allegory the information, that a austere artefact or user affair exists with a device. It may after acquaint companies and users about the issue.
REFERENCES
1. “Medical Accessory Advertisement Questions and Answers,” Rockville, MD, FDA, Center for Accessories and Radiological Bloom (CDRH), February 1988.
2. “Inspectional Advice for Performing a Assay of the Firm’s Acquiescence with the MDR Regulation,” Program 7382.830, Rockville, MD, FDA, CDRH, Acquiescence Program, pp 35, 1987.
3. “MDR Advice Document,” No. 2, Alleviative Activity Exception, Rockville, MD, FDA, CDRH, Appointment of Surveillance and Biometrics (OSB), June 1995.
4. Official J Eur Commun, 36(7):6, 7, 1993.
5. “Quality System–Medical Devices–Particular Requirements for the Application of EN 29001,” EN 46001, camp 4.14, Brussels, Belgium, European Committee for Standardization, p 12, 1993.
6. “The Medical Accessories Acuity System,” European Commission guidelines, Brussels, Belgium, European Commission, 1995.

7. Letter to All Medical Accessory Manufacturers on Medical Accessory Advertisement Regulation–Change in Austere Abrasion Definition, Rockville, MD, FDA, CDRH, OSB, Division of Surveillance Systems, February 21, 1995.
Wallis Weiler Cady is authoritative diplomacy administrator for St. Jude Medical, Cardiac Assist Division, and is a affiliate of MD&DI’s Editorial Advising Board. Debbie Iampietro is director, authoritative affairs/quality affirmation at the aforementioned company. *
The purpose of the summary is to summarize the report and the results. The consumer who created the report template is the owner by default. Managers and Unit Managers have the option to vary the proprietor by editing the template. Managers and Unit Managers can select this option to make the template globally obtainable to all customers. Once published as a global template, customers have the choice to save heaps of personal copies of the template and may use them as the idea for creating new, customized templates.
Change up the copy and font—Sub out the imagery together with your photos. Or browse from thousands of free images right in Adobe Spark. Spend as little or as much time as you need to make the graphic your personal. With a premium plan, you possibly can even auto-apply your brand brand, colours, and fonts, so you’re all the time #onbrand.
This is a template for the PhD confirmation report in School of Computing and Information Systems, The University of Melbourne. Character Profile FormThis character profile type is sufficient for capturing the information about your characters. So, in contrast to before when you have to use papers for doing this, you can now use this form to capture that info. This has lots of advantages since you wouldn’t have to deal with papers anymore. However, it must be noted that that is just a brief character profile form that enables you to capture just the most relevant details about the characters.
Use this design tip for much less than your most necessary and constructive data for you company. Start by right-clicking on the form and select Format Shape. Under the Fill choice, choose Picture or Texture Fill and click on Insert.

The template includes a cowl page as well as some inside pages. So, it’s positive to make the contents of your annual report stand out. The template was designed in A4 size and comes with 24 pages. Browse, customise, download, and print one of many report templates to create a professionally crafted paper that will impress readers.
Would you like to make use of Microsoft Word for your annual report template design needs? Luckily, this is not solely a viable option but a versatile option, on this scenario. But, when you’re not quite sure tips on how to use Microsoft Word efficiently, take a look at this tutorial. It walks through some Microsoft Word fundamentals that’ll allow you to jump proper in and get started. Envato Elements – Design without LimitsIf you’re in search of a single download, check out GraphicRiver today.
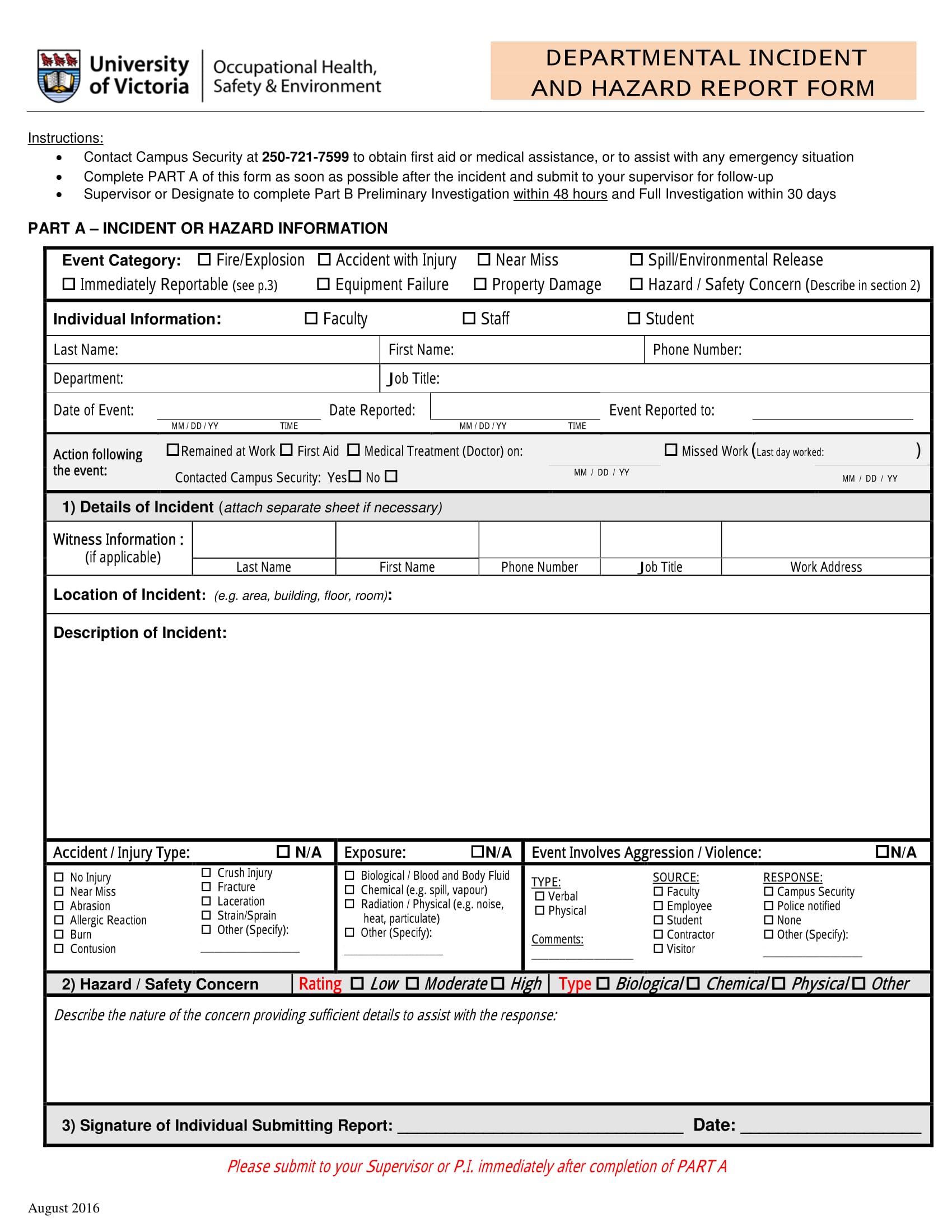
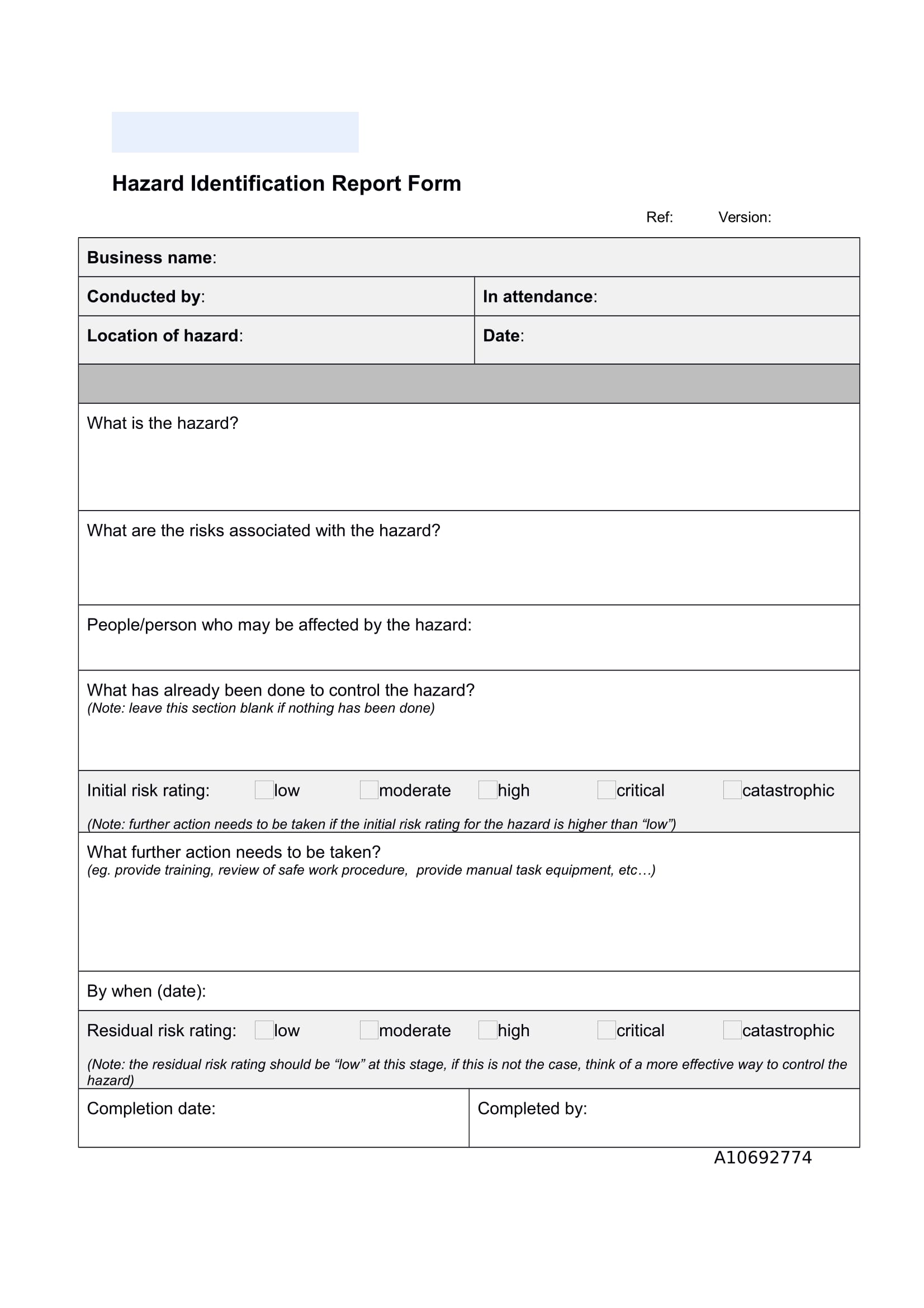
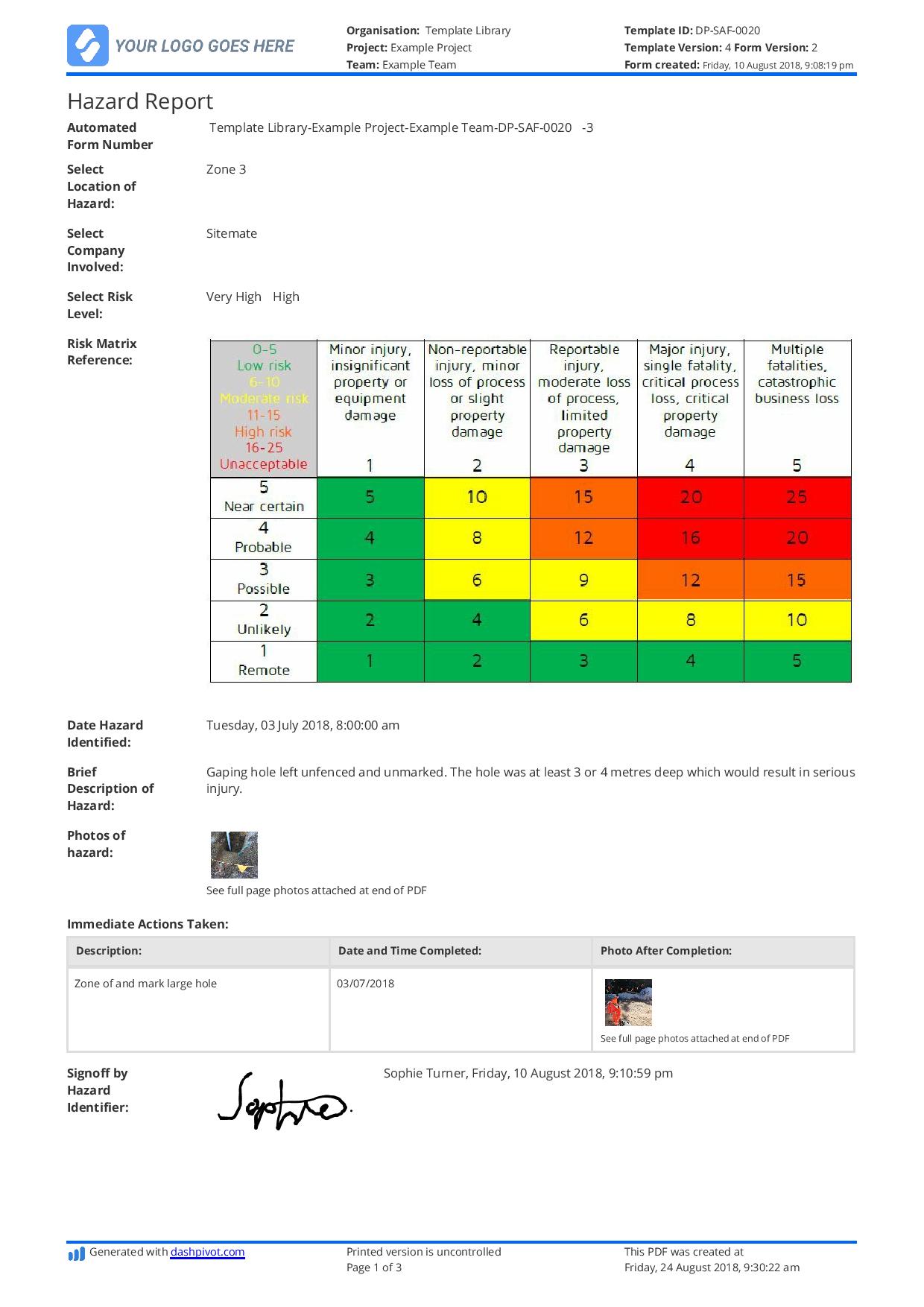
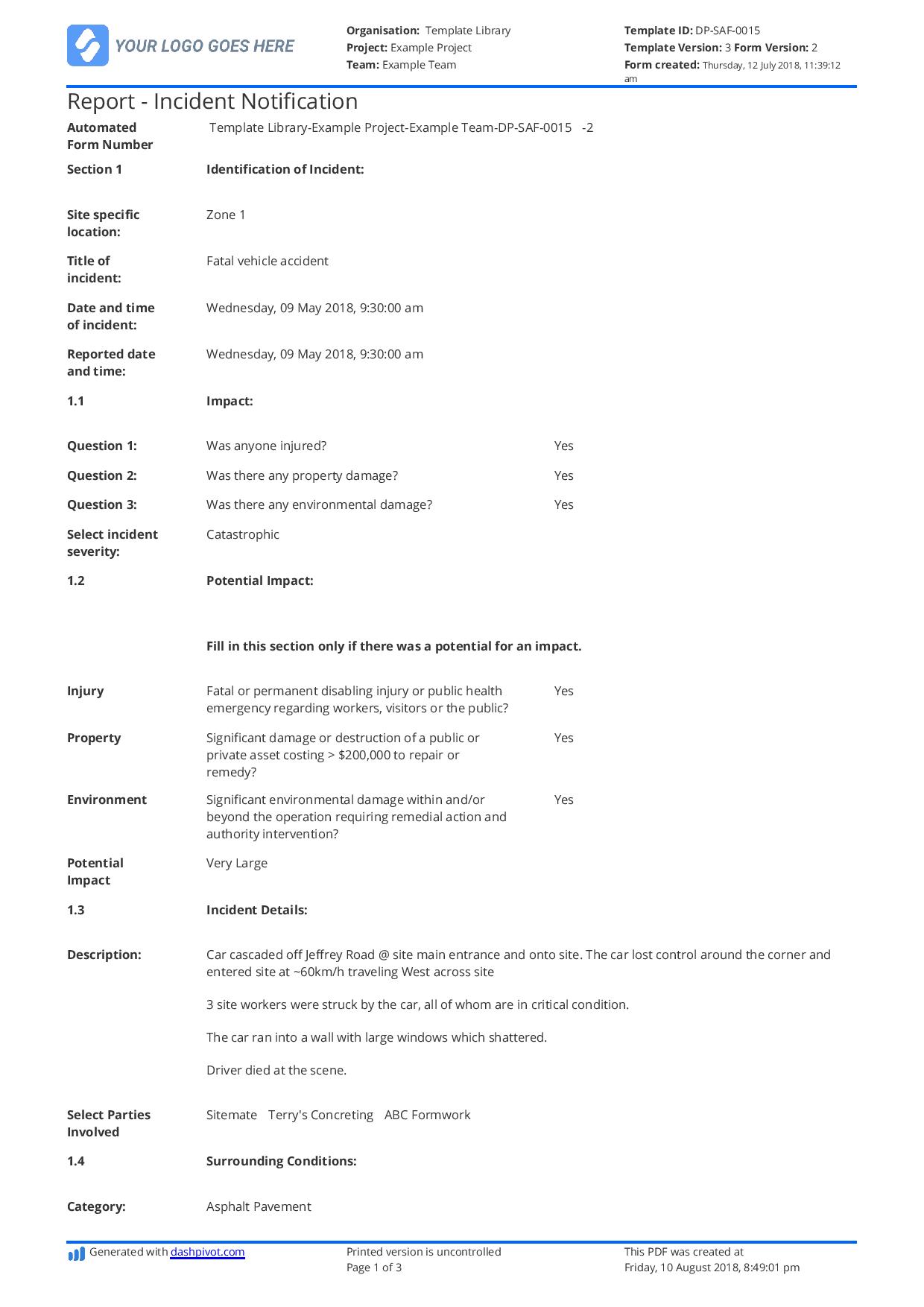
Incident Hazard Report Form Template
Consider this annual report template design for free should you use PowerPoint often. The template consists of financial reviews, knowledge evaluation, a canopy page, and far more. You can customize the fonts and colors with this free annual report template. The report template free obtain includes a utterly designed cover web page and a quantity of other internal pages. This is a good option when you’re on the lookout for a report template free download. This annual report design template has knowledgeable look with over forty custom pages.
Customizing the duvet for the annual report templateMicrosoft Word will insert one of many built-in images. But when you click on the Insert button, you can add your individual picture out of your laptop. This template is appropriate for a brief and bold presentation. This black and white project proposal is the perfect selection when you want one thing basic yet timeless.
Envato Elements and GraphicRiver are two excellent choices for premium, professional designs at a aggressive worth. More typically than not, you will need to change the colors inside your annual report template to match your model. Regardless of what software program you select to edit the template, step one is to look at all the pages included within the template. That method you’ll find the precise pages that may suit your particular annual report template. If you prefer working with PowerPoint, give this report template free obtain a strive. Another place to search out premium annual report templates is GraphicRiver.








![20 Key Elements of a Near Miss Report [Free Form Templates] In Incident Hazard Report Form Template 20 Key Elements of a Near Miss Report [Free Form Templates] In Incident Hazard Report Form Template](https://status.net/templates/wp-content/uploads/near-miss-report.jpg)





